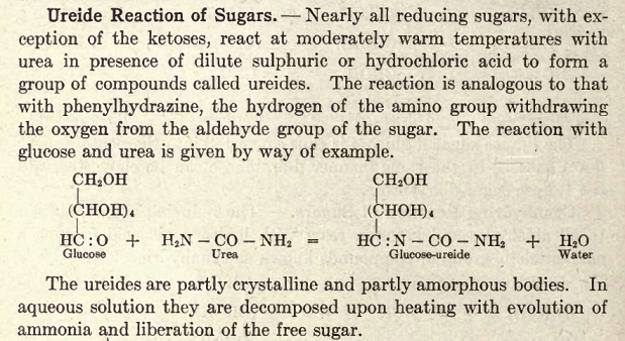Im surprised that this compound hasn't taken off, has anyone tried making any glucose ureide?
I tried the synthesis as follows
10.2g of glucose hydrate dissolved in 3g of water at 60c.
34g of urea is added and stirred in.
When the temperature is 60c again, 91.8g of glucose hydrate is added and also stirred in.
Afterwards, once the mass is mostly melted, a mixture of 3g of water and 3g (~1.6ml) of sulphuric acid are added slowly.
The mixture reacts at 60c for 8 hours.
Here, no solid glucose ureide was noticed, however the black viscous mass was obtained, but I continued with the synthesis as is written in the
patent.
The mix was dissolved in very little water and neutralised with barium carbonate.
This was then filtered and evaporated, however no precipitate was obtained.
I have repeated this procedure but allowing a reaction time of 16hours, but with the same result.
Has anyone managed to make any glucose ureide yet? |




 It doesn't say "increased to"
It doesn't say "increased to"