
Σldritch - 18-8-2023 at 01:18
I have become fascinated with 1,8-dihalobiphenylene derived catalysts. Theoretically a whole range of interesting catalysts would be easily available
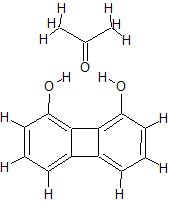
if you just had this precursor available. There is of course 1,8-biphenylenediol which activates carbonyl groups but slap some
guanidine/triaminocyclopropenium on that skeleton instead and you should end up with a organocatalyst for the Wolff-Kishner reduction. There are many
more possibilities of course and I have some more 'hopeful' ideas already.
The question is just how to make it. The paper below mentions that 1,8-dibromobiphenylene can be made by the Ullmann reaction of
2,2',6,6'-tetrabromobiphenyl. While I would hope for a method to directly produce this precursor from biphenyl - itself readily made - I am not so
hopeful. Any byproducts would be quite nasty, I understand, though apparently this target isomer is suposed to be less so according to Wikipedia...
So my initial idea is this: Toluene -> p-tosylic acid -> 2,6-dibromotosylic acid -> 2,6-dibromotoluene -> 2,6-dibromobenzoic acid ->
2,2',6,6'-tetrabromobiphenyl -> 1,8-dibromobiphenylene.
Nothing crazy each step, though there are a lot of them, am I thinking correctly here? Though, surely there is a better way, right? Anyone?
EDIT: Looking at the organic chemistry portal I find that Bromine catalyses the oxidation of methyl-arenes to aryl-carboxylic acids. With the right
brominating agent a lot of steps should then be able to be combined making the steps: Toluene -> p-tosylic acid -> 2,6-Dibromobenzoic acid ->
... as the hydrolysis of the sulfonic acid should be possible in the same step.
Attachment: RecentAdvancesOfBiphenylene.pdf (2.6MB)
This file has been downloaded 193 times
[Edited on 19-8-2023 by Σldritch]
