
vano - 6-9-2022 at 10:55
Hello! I had some molybdenum powder, so added conc. hydrochloric acid, some of them dissolved, the solution has green color, then I added
phenanthroline and it turned red like iron(III). so I made more con. solutions and precipitate this complex compound. I know Mo(III) is rare, but as I
found some research, its phenanthroline complex does exist. so this very dark red compound is the result.
in live, it really has a red color, ill take a picture under sunlight tomorrow.




[Edited on 6-9-2022 by vano]
Attachment: Mo(III).pdf (305kB)
This file has been downloaded 290 times
Bedlasky - 6-9-2022 at 19:01
Mo dissolves very slowly in HCl to form Mo(V). The green complex is [MoOCl5]2- ion. So your phenantroline complex is bound to Mo(V), not Mo(III).
vano - 6-9-2022 at 22:33
How this structure looks like what you think?
vano - 7-9-2022 at 00:31
new photo:

Bedlasky - 7-9-2022 at 22:44
Molybdenum form usually six-coordinated octahedral complexes. Your complex probably contain Mo=O group with two phenantrolines and one H2O or Cl. But
this is just guess, it could be coordinated on Mo2O4 or Mo2O3 group. If you want to know true composition, you must do some analysis.
[Edited on 8-9-2022 by Bedlasky]
vano - 18-9-2022 at 03:37
thanks, Bedlasky! I made the structure you mentioned. now I can but I'll make some analysis.
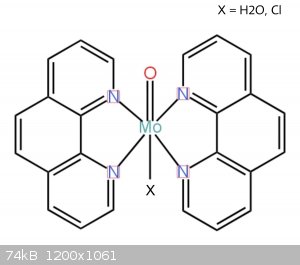
[Edited on 18-9-2022 by vano]
vibbzlab - 18-9-2022 at 03:53
I have made a similar complex long back
https://youtu.be/MdiMPaOx98w
It's a phenanthroline, vanadium complex
vano - 18-9-2022 at 04:44
Yes, I have seen it maybe a year ago. interesting complex.
