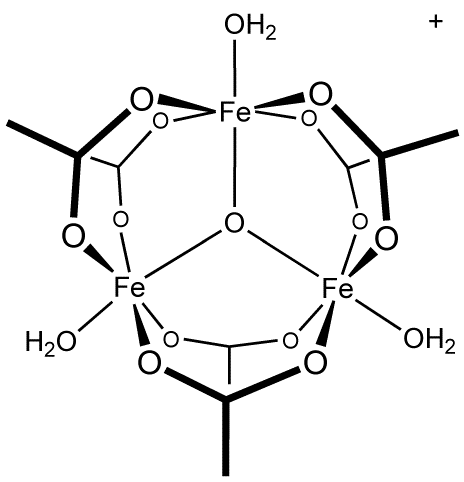
| In the case of the central oxygen, it doesn't have three full single bonds. It's technically three "2/3 bonds" for a total bond order of 2 for oxygen,
as usual. Bonding can get really weird when you get into delocalized electrons. We usually talk about whole-number bond orders because it's
impractical to draw fractional bonds, but sometimes electrons can be delocalized (shared across more than one bond), which leads to fractional bonds.
The most common example is benzene, which contains 6 equal length "1.5 bonds" rather than alternating single and double bonds as it is often drawn.
|