
metalresearcher - 30-12-2021 at 12:30
I found on "The Action Lab" Youtube channel a video about crystallizing sodium acetate (CH3COONa) which can be supercooled.
As I have 'cleaning vinegar' I repeated this experiment. I neutralized some of it with Na2CO3 until CO2 release stopped. Then I evaporated it on an
evaporating dish while still keeping liquid. Then I allowed it to cool in the beaker to let it supercool.
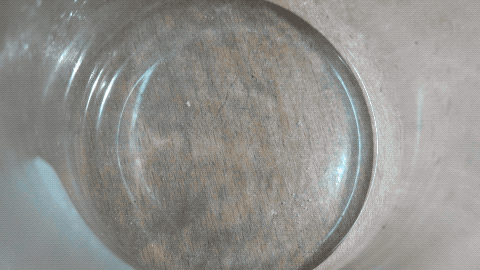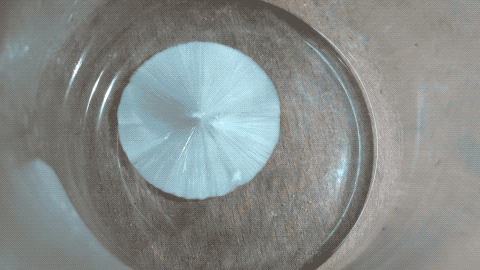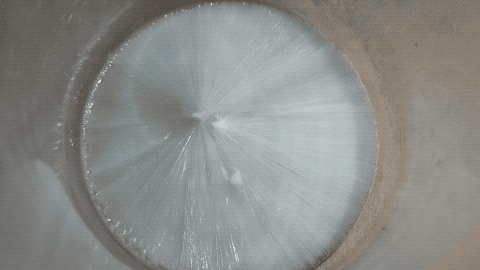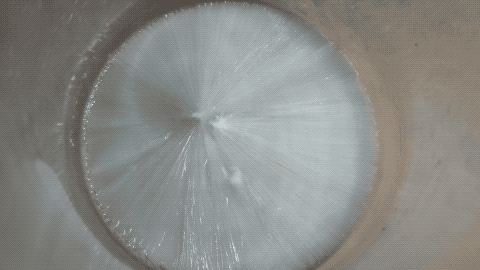
Then I put a tiny grain of Na2CO3 in it and then it crystallized quickly.
Note: this is not a time lapse, but real time ! I was surprised as I thought the YT video was a time lapse while it is actually a real time video.
Bedlasky - 30-12-2021 at 13:08
This is one of my favorite experiments. Simple, but still quite spectacular. When you take the beaker to your hands, you can feel heat, which is
released during crystalization. Or you can make your own "stalagmite" when you pour supercooled sodium acetate on a surface.
Lion850 - 30-12-2021 at 14:52
Beautiful!

