
briankim78 - 9-3-2011 at 19:35
Dear all,
I am preparing some chemical which is very reactive to the atmosphere.
Therefore, I am trying to put the material inside the glass ampoule and seal it while it is prepared inside the glove box.
The glove box I use is filled with Argon.
Any suggestions?
[Edited on 10-3-2011 by briankim78]
Fleaker - 9-3-2011 at 19:47
Eh...I'd put it into a schlenk flask that has a glass or PTFE high vacuum seal and then I'd distill or vac transfer to the ampoule (sealed to the
line) immersed in liquid nitrogen. You can calibrate the ampoule to hold a specific volume. This is what I did when I did polymerizations--I'd send
over inhibitor free, de-gassed monomer and then seal it. That would get immersed in an oil or salt bath.
What's the chemical?
briankim78 - 9-3-2011 at 20:11
Well, they are iodide compounds. (ScI3, DyI3, CsI3...)
Up until now, I put them inside the vial then put a plastic cap.
After that I took it out of glove box for sealing.
Just before I place the vial on the sealer, I made small pin hole on the plastic cap so it does not bust.
I thought this was quite safe think to do.
But some of my collegues told me that "STILL" there is a huge chance for contamination by air.
They just left a huge homework on me and no answers not even any suggestions!!!
Gee~!!
Does anyone have idea on plasma sealing or what ever?
They told me that some lamp manufacturers are using plasma to seal-off their quartz or glass tubes inside the glove box.
I'm very curious and want to know how to get one.
I need your helps~!!!!!!!!!!!!!!!!
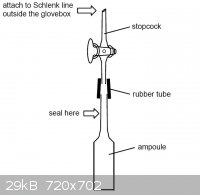
matei - 10-3-2011 at 09:47
Just attach a piece of vacuum rubber tube with a stopcock to the ampoule after its filled, get it out of the glovebox and than attach it to the
Schlenk line, evacuate it and than seal it with a torch.
[Edited on 10-3-2011 by matei]
itchyfruit - 10-3-2011 at 13:07
I've often wondered had they did this with caesium and such like.
