vibbzlab - 17-4-2021 at 05:47
Hey guys
So I have some interesting compounds like
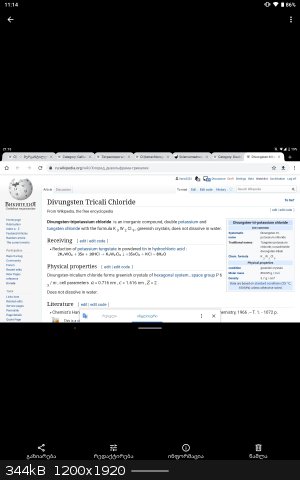
Sodium tungstate, sodium molybdate , V2O5 , ammonium metavanadate, bismuth salts , cesium chloride.. etc
Can you guys suggest the various colored complexes that I can make using ligands like en , acetyl Acetone etc. Or even double salts. Any ideas are
welcome.
I just want to make some interesting compounds
Thanks in advance
vano - 17-4-2021 at 06:48
If you write more substances it will be much easier to think. For example, do you have selenium? What metals do you have? Gallium, indium others?
•You can make organic tetrathiovanadates (NR4)3VS4.
•vanadyl hexacyanoferrate (VO)2[Fe(CN)6], anhydrous is green.
•molybdates and tungstates have very beautiful colour, but if its boring you can make phosphotungstates & molybdates, silicomolybdates &
tungstates, you can use arsenic acid and many other acids. Many of them are colourful.
•Bismuth oxychromate
I can not think of anything more now. For example you can make vanadyl acetylacetonate from that chemicals, but you already know that.
vibbzlab - 17-4-2021 at 07:10
Ok I will note down the ions that I havw
Vanadium
Chromium
Copper
Cesium
Cerium
Molybdenum
Tungsten
Bismuth
Then ofcourse the common things
vano - 17-4-2021 at 08:06
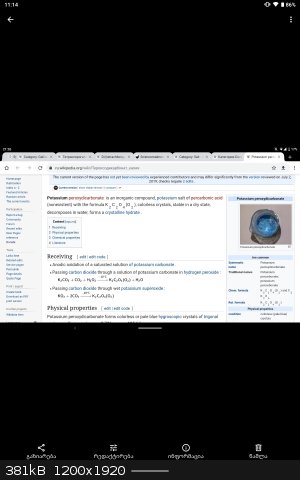
•Tetraamminezinc tetraperoxomolybdate is red-brown solid.
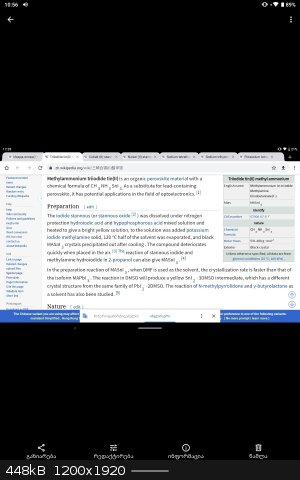
•Methylammonium triiodostannate is perovakite material, but it has dark colour.
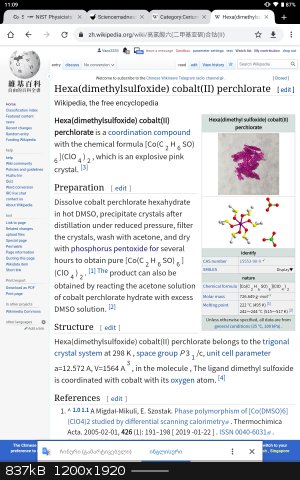
•you can make DMSO complexes for example SnI2•3DMSO it is yellow, PbI2• 2DMSO and other metals complexes.
• barium oxohydroxostannate(2) is yellowish Ba[Sn2O(OH)4]
• dinytrosyl cobalt halides. (NO)2CoCl Etc.
•Tris(cyclopentadienyl) cerium is orange.
• complex salt Potassium tetrafluorocerate (III), i never seen other halogens instead of fluorine.
DraconicAcid - 17-4-2021 at 09:13
With copper, you can make ammonia complexes (purple-blue), as well as Cs2CuCl4 (yellow).
vibbzlab - 17-4-2021 at 17:12
If you guys can provide me with the preparation notes it will be very helpful.
vano - 17-4-2021 at 23:17



http://www.sciencemadness.org/talk/viewthread.php?tid=156840...