
Chem Science - 11-4-2020 at 20:29
Greetings everyone !!
If there were a synthesis that i say sparked my interest in chemistry, is the making of Luminol. NurdRage released his Luminol Synthesis video 9 year
ago [NurdRage's Video] an i remember seeing it and being so amazed.Generating light from chemistry was totally awesome, since then i wanted to make it
my self. And here we are .. 9 years in the future and i can say my dream has finally been accomplished. Making Luminol was work well enjoyed, up to
the 3-Nitrophthalhydrazide there was no problem, but the reduction was a problem.Here in Argentina Acetone is IMPOSSIBLE so NurdRage's method was not
possible for me. UC235 did the reduction with Thiourea Dioxide [UC235 VIDEO], also impossible,NileRed did it with Dithionite [Nile's Video], also impossible.In general reduction of nitro compounds are difficult for the Argentine amateur chemist.
I had 2 options for the reduction. The Zinin reduction and the Piria reaction. I did the Zinin reaction in NItrobenzene and made a post of that [LINK HERE]
In these post i will detail the Piria reaction (Only 1 experiment) and the Zinin reduction (More detailed) in 3-Nitrophthalhydrazide.
REAGENT QUALITY
Sodium Sulfide 60%: Lab Grade (Pro-Analisis)
Ammonium Sulfate: Tech Grade
Sodium Hydroxide: Lab Grade (Min.98%)
Hydrochloric Acid: Tech Grade (Colorless)
Ethanol: Pharmacological grade 96%
3-NitrophthalHydrazide: Synthesized
Reduction of 3-Nitrophthalhydrazide to Luminol
PIRIA REACION
INTRODUCTION:
The Piria reaction is a Reduction Reaction for aromatic nitro compounds by the use of Sodium Sulfite – Bisulfite in aqueous solution. These reaction
is useful for the amateur because it produces an aromatic amine along with an aromatic amine-sulfonic acid. So if for instance you start with
Nitrobenzene, you end up with Aniline and p-Amino Benzene Sulfonic acid. The procedure of the Paper [1] uses solutions of sodium bisulfite and
hydroxide that has to be mix before the reaction, in my case, I instead use sodium Metabisulfite and sodium sulfite. These is faster as you only need
to dissolve the salts and you’re done. I won’t go in more details as I did some experiments with Nitrobenzene to get Aniline and Sulfanilamine and
I will talk more in another tread. These was just 1 try at these reaction.
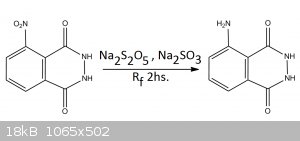
EXPERIMENTAL PROCEDURE
In a Reflux apparatus were mixed 1g of 3-Nitrophthalhydrazide along with 3,671g of Sodium Metabisulfite (Na2S2O5) and 0,912g of Sodium Sulfite
(Na2SO3) and approximately 25mL of water. These mixture is put to reflux for 2hs. During these time the Nitro-compound dissolves gradually and
completely. After the 2hs the mixture was allow to cool and acidified with 7M Hydrochloric acid (Addition of the acid did not produce appreciable
amounts of sulfur dioxide) Luminol precipitated slowly after cooling, and as a very fine powder. Filtration and drying gave 190mg (22, 2%) of luminol.
The rest of the solution, after neutralized, upon addition of bleach (Considerable amount) gave strong Chemiluminescence, these does not prove but
gives some clue, that some soluble sulfonate of luminol may be formed. No attempt to recover these was done. (Maybe saturating with NaCl to
precipitate the sodium salt?)
ZININ REACTION
INTRODUCTION:
The Zinin reaction is a reduction reaction for aromatic nitro compounds, is useful because it can be used to reduce 1 aromatic nitro group in
compounds that have 2 nitro groups in the same aromatic ring. I have a Sciencemadness post of these reaction with Nitrobenzene to make aniline [Zinin Reactin on Nitrobenzene]. There is a paper [2] on the use of these reaction for the synthesis of luminol and claims up to 64.5% yield!! In
the paper they use Ammonium sulfide, I don’t have ammonium sulfide, so I replace it with sodium sulfide and ammonium sulfate instead. (The ammonium
sulfate is important, go to the Comments to see why)
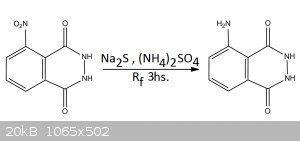
EXPERIMENTAL PROCEDURE
USING SODIUM SULFIDE LAB GRADE
In a reflux apparatus was added 3g of 3-Nitrophthalhydrazide, 9g of Sodium sulfide and 30mL of water Approx. The mixture turns dark red as the
alkalinity of Na2S dissolves the nitro compound, these was put to reflux for 3hs (The color doesn’t change that much). After reflux the mixture is
acidified with 7M HCl while hot, SH2 is liberated, and precipitation of luminol and sulfur occurs, the solution is placed in the freezer to cool.
After cooling the precipitate is filtered, and while slightly wet it’s placed in a beaker and luminol extracted with hot ethanol in 2 portions,
100mL and 50mL respectively. Filtration of the hot ethanol separates the undissolved sulfur. Cooling of the ethanol precipitates luminol, filtration
and drying gives 179mg (6.8% yield) of Luminol contaminated with some sulfur. I know... These sucks, in the comment section you will see why these
happened.
USING SODIUM SULFIDE LAB GRADE AND AMMONIUM SULFATE
In a reflux apparatus was added 1g of 3-Nitrophthalhydrazide, 1.884g of Sodium sulfide, and next 25mL of water, next is added and with stirring, 2g of
ammonium sulfate are added (by portions with a spatula). After the ammonium sulfate is added the mixture is reflux for 3Hs. At the 11⁄2hs a solution
of 2g more of ammonium sulfate in 10mL of water is added slowly through the condenser, and the reflux continue. After the 3Hs the heat is turned off
and the mixture cooled. Acidification with 7M HCl precipitates Luminol ans Sulfur which are vacuum filtered and the luminol separated from the sulfur
with hot ethanol (75mL in 2 portions) and filtration. Cooling of the ethanol precipitates luminol. Final yield was 286mg (33,45%).
USING SULFUR, SUDIUM HYDROXIDE AND AMMONIUM SULFATE
In a reflux apparatus was first mix 6,6g of regular sulfur and 6,2g of sodium hydroxide along with 50mL of water. These mixture was heated and stirred
until all, or most of, the sulfur dissolves, when these happened 1g of 3-Nitrophthalhydrazide was added flowed by 5g of Ammonium Sulfate (Added
slowly). These mixture was reflux for 3hs, at the 11⁄2hs. Of reflux a solution of 5g of ammonium sulfate in 15mL of water was added slowly through
the top of the reflux condenser. During the 3hs of reflux, the solution re-precipitates nitrophthalhydrazide, which re-dissolves again, the solution
is always a dark color and has some sulfur precipitated at the end. After the 3hs the mixture was allow to cool, and acidified wit 7M HCl, these
precipitates sulfur and luminol which are suction filtered, and extracted with hot ethanol (100mL In 2 portions) to separate the Luminol and sulfur.
Cooling the ethanol precipitates luminol. Final yield was 448mg corresponding to 52.3%.
COMMENTS:
First of all... during the reflux of the reaction A LOT OF THINGS HAPPEN... a solid forms in the condenser (maybe ammonium sulfide?), some yellow
liquid refluxes (WHTF .. yes .. but it happens !!)
It might look as if the “Crude” amateur second procedure is better, but in fact I made a HUGE MISTAKE in the first procedure. I did the reaction
about 3 times using pure lab grade sodium sulfide, and it was at the 3rd time I realize I was an idiot. The yield of my 3 attempts was always bad and
I couldn’t understand why if the paper claims more than 60% yield, I even used a 3g run and still got it wrong, so I check stoichiometry and was Ok,
check reactions time and was ok … I was using sodium sulfide instead of ammonium sulfide and the Sulfide was the important ion, I had good yield
with nitrobenzene in similar conditions in the past so… what was wrong??? It was when I check the mechanism of the reduction that I realize … yea
I am a total idiot. The ammonium ion in the ammonium sulfide is actually an important component!
The proposed mechanism for the reduction of NITROBENZENE is the following
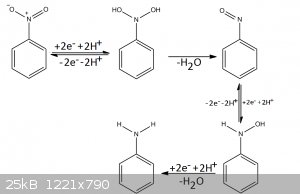
Yea I know these is not 2-Nitrophthalhydrazide but the mechanism is the same, the important detail is that for the reduction to occur
there must be protons! Ammonium Sulfide is 1000 times more acidic than sodium sulfide, these is because Ammonium ion has the following equilibrium in
solution.
NH4(+) + H2O ↔ NH3 + H3O(+)
(I know the symbol is not of equilibrium, I didn’t find the symbol in word xD xD)
So pH was the problem … Yea these is classic amateur mistakes (or maybe it’s just me xD feel free to criticize) once I added the Ammonium sulfate
as a source of ammonium ions the reaction was more efficient. Unfortunately from the 8,5g of 3-Nitrophthalhydrazide I made in 1.5 weeks of work I used
6 of theme in failed attempts... I feel an idiot but it’s nice to know at least I realize my mistake. What’s also nice is that the procedure with
sulfur work’s and is a nice way to make the reduction, I can’t find sodium dithionite or thiourea dioxide to do the other more classic reductions
so I had to try these ones.
A note on the Luminol Purity and purification, using ethanol is actually a bad idea in the first place, as some sulfur does dissolve
and precipitates along with luminol. The best purification is to add small amounts of sodium carbonate (Na2CO3) while cool and check the pH, filter
off the sulfur and re-precipitate the luminol with acid, I did not do these because the first time I tried it I had a bad luminol yield and also bad
quality so that kind of pushed me away from the procedure. But at the end I got all my Luminol from the attempt’s and did the purification wit
carbonate and went well with nice and yellow luminol as the final product.
REFRENCES:
[1]"The Preparation of 3-Aminophthalhydrazide for use in the Demonstration of Chemiluminescence"
[2]"The Piria Reaction. II. Mechanism Studies"

 Attachment: ETHANOL EXTRACTION (156kB)
Attachment: ETHANOL EXTRACTION (156kB)
This file has been downloaded 438 times Attachment: ACIDIFIED REACTION MIXTURE (88kB)
This file has been downloaded 433 times Attachment: LUMINOL YIELD (138kB)
This file has been downloaded 429 times Attachment: MIXTURE BEFORE REFLUX (119kB)
This file has been downloaded 426 times Attachment: MIXTURE AFTER REFLUX (184kB)
This file has been downloaded 463 times Attachment: REACTION AFTER 3Hs REFLUX (78kB)
This file has been downloaded 426 times Attachment: YELLOW STUFF IN THE CONDENSER (113kB)
This file has been downloaded 433 times Attachment: LUMINOL YIELD (144kB)
This file has been downloaded 445 times
[Edited on 13-4-2020 by Chem Science]
[Edited on 13-4-2020 by Chem Science]
mackolol - 12-4-2020 at 03:16
Very nice workup, I did my reduction with thiourea dioxide and it was very clean, cheap and easy.
Of course, if you're not able to purchase thiourea, it would be practically impossible, as synthesising it is very stinky and dangerous.
Chem Science - 12-4-2020 at 06:38
Thanks mackolol 
The only place i saw Thiourea was on my Bosse's lab in university. It's a nice reagent but out of my reach for the present time 
I think the Zinin reaction has potential  but i screw up
but i screw up  and couldn't do more than 2 actual representative experiments. xP
and couldn't do more than 2 actual representative experiments. xP
UC235 - 12-4-2020 at 09:37
Thiourea dioxide is used for fabric dyeing as "color remover." It has a number of other names like formamidine sulfinic acid. It is considerably more
available than thiourea itself. Some of these color remover products are sodium hydrosulfite, which also works. I am surprised that none are available
at all.
Chem Science - 12-4-2020 at 09:50
Oh my .... It's an honor UC235 !!
Well i did little search in the area of Thiourea Dioxide, i think it's time to research again to see if i can find it, meaby i really just did not yet
looked good enough  . I'll keep looking
. I'll keep looking 
karlos³ - 13-4-2020 at 09:49
Thats prepublication worthy, very cool!
Chem Science - 13-4-2020 at 13:48
Thank you very much karlos³ 
I still need to work on the narration a little xD said too many times "Added" xD hehehe 



 but i screw up
but i screw up  and couldn't do more than 2 actual representative experiments. xP
and couldn't do more than 2 actual representative experiments. xP . I'll keep looking
. I'll keep looking 

