
Arcaeca - 12-9-2019 at 22:14
Another idea that occurred to me for making red pigment: the blood-red resin of Dracaena cinnabari, the dragon blood tree, which grows on the
island of Socotra off the coast of Yemen, has long been used as a wood varnish and, in its solidified form, a red pigment. According to this paper (https://www.academia.edu/22733111/Identification_of_7_4_-Dih...), the red pigmentation mainly comes from the compounds dracorhodin,
nordracorhodin, dracorubin and dracoflavylium - all of which are flavanoids and are pictured a couple pages into that paper.
I did find one paper (https://pubs.rsc.org/en/content/articlelanding/1950/jr/jr950...) that gives a synthesis for dracorhodin, but it's quite hard to follow; it's not
entirely clear what their starting material is, and at one point they say all their attempts to do one part of the synthesis were unsuccessful... and
then they continue on as if it did work after all?
Further, I'm tentatively going after specifically dracorhodin, because apparently it can dimerize (spontaneously?) to give dracorubin (https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201705...), so I might end up getting 2-for-1. But ultimately I don't really care
which chemical it is as long as it has the same color and properties as dragon blood resin.
Now, since anthocyanins are also flavanoids and can be found in all sorts of fruits and flowers and thus fairly easy to get, I figured I might see if
I can prepare dracorhodin from anthocyanins. They're fairly easy to extract with methanol (or from grape skins in dilute aqueous tartaric acid: https://www.researchgate.net/publication/51995381_A_method_f...). The question is then... how to convert them to dracorhodin?
Because I haven't been able to find anything about either the biosynthesis or a total synthesis of dracorhodin - I don't know if it's feasible to
strip an anthocyanin down to a flavylium cation and then rebuild it up to dracorhodin? If so, I guess the first step would be to deglycosylate the
anthocyanins (which I don't know how to do; I requested the full text of this paper (https://www.researchgate.net/publication/324179780_The_conve...) but have yet to hear back from the authors), but then... what next? Is there an
easier way to turn anthocyanins into dracorhodin? I'm guessing that's a question nobody knows the answer to, but does anyone have an educated guess?
Arcaeca - 16-9-2019 at 20:37
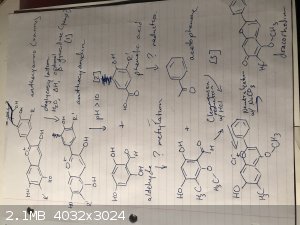
Okay, so after a couple more days of trawling through papers, I think the attached, nearly complete pathway from anthocyanins to dracorhodin.
Anthocyanins are deglycosylated to anthocyanidins by heat, soaking, and β-glucosidase [1], which I think I might be able to get from yeast. Then
anthocyanidins break apart into an aldehyde and a phenolic acid at pH 10 - 11 [2]. A specific aldehyde very similar to the one made from the
anthocyanidins, and acetophenone can undergo a Clemmensen reduction in HCl, and then the acid is neutralized to give dracorhodin [3].
The problem is those mystery lines. The general anthocyanidin aldehyde needs to undergo methylation twice - once to turn an alcohol group into an
ether, and once on the main ring itself - and to turn the phenolic acid into acetophenone would require two reductions - one to turn the carboxylic
acid into a ketone, and one to strip off an alcohol group.
Attaching a methyl group to an arene can be done with FC alkylation IIRC, but I can't stop it from attacking the phenolic acid too. (Plus it would
require methyl chloride as the methyl group donor, and that's not exactly sold in grocery stores) Going back through my o-chem notes I can't find
anything about turning an alcohol into an ether, and I have notes on reducing carboxylic acids... with reactants like lithium aluminum hydride that
will reduce not just to a ketone, but all the way to an alcohol.
Anyone have any ideas what reactants might make it work?
[1] https://doi.org/10.1016/j.foodchem.2018.03.152
[2] https://doi.org/10.2021/jf900602b
[3] https://doi.org/10.1039/JR9500001882
[Edited on 9-17-2019 by Arcaeca]
Tsjerk - 17-9-2019 at 12:38
I had a look. I think the de-glycosylation could work.
From there on: could you put the mechanisms in a more 2D and more clear way? I love to help you but next to my daily job this is to much to figure
out.
