
trb456 - 21-1-2011 at 11:08
I'm a recent lurker, just joined--fun site!
I'd like to perform a very small scale halogen experiment based upon part of Experiment 11 from Laboratory Manual for Principles of General
Chemistry (link). In brief, acidify halogen salts and capture released elemental halogens (Cl, Br, I) in cyclohexane, then use to show differing
reactivities. Pretty straightforward, except that cyclohexane is fairly impossible to order in the US as a hobbyist. Any suggestions for an
alternative? I was thinking toluene, and will probably just test this on Lugol's to see if it works. But is there another common nonpolar solvent
that someone knows would be better for this, or knows why toluene could be a problem? Thanks in advance!
trb456
Magic Muzzlet - 21-1-2011 at 11:13
Cyclohexane is very easy to obtain especially in the us, even as a hobbyist. But I would use DCM probably as a replacement, but then again I do not
know exactly what the experiment is.
trb456 - 21-1-2011 at 11:39
Thanks for the quick reply! Every online site I checked (admittedly not very many) either did not sell or only sold to commercial/schools.
I don't have DCM, but I do have chloroform. I saw that might work well, too.
Experiment is simple: extract various halogens by acidification, and capture in the solvent. So I just need good and safe solubility.
NaClO + 2HCl -> Cl2 + NaCl + H2O
2KBr + 4HNO3 -> Br2 + 2H2O + 2NO2 + 2KNO3
2KI + 4HNO3 -> I2 + 2H2O + 2NO2 + 2KNO3
trb456
Magic Muzzlet - 21-1-2011 at 11:50
Chloroform should work just as well for your purpose I presume. For cyclohexane elemental scientific has it, as well as chemsavers. Many other sources
as well, finding chemicals is a game all in itself, but just about anything can be procured, trust me 
DDTea - 21-1-2011 at 17:53
Cool experiment. Br2 in methylene chloride is actually a useful reagent. I'd sooner use MeCl2 over chloroform just because it's cheaper.
ThatchemistKid - 21-1-2011 at 18:34
In the U.S. DCM is pretty easy to get (assuming you have a distillation set up of some sort). Just buy some klean strip paint stripper from home
depot, walmart. Look for the bottle that does not say that it is flammable on it, says it contains methylene chloride and methanol (and nothing else)
I found that this was the 92% DCM 8% methanol azeotrope that distills around 38 degrees C. The solution comes mixed with a small amount of paste that
thickens the whole thing, just distill it off of that and wash the paste out of the flask with some acetone and hot water.
after distilling the azeotropic mix, wash with water to get out the methanol and re-distill the DCM. For 9 dollars I got 700 mls of the stuff which i
think was a pretty great deal.
trb456 - 25-1-2011 at 11:34
ThatchemistKid, thanks for the info. I just checked the MSDS for the stripper, and it looks like the paste is white spirit with a much higher boiling
point (130C+). You say the DCM and CH3OH form an azeotrope and we need to 'wash" out the CH3OH. Just to be clear, are you saying that H2O and CH3OH
preferentially mix and form an even higher boiling point azeotrope than the DCM, thus allowing the DCM to be distilled off? If this is all correct,
could we not simply wash the stripper at the start and distill off the DCM immediately, leaving the H2O, CH3OH and white spirit behind? Or, are you
saying wash with H2O and extract the H2O/CH3OH mix before distilling again, in which case, why not just wash/extract initially and distill off the DCM
leaving white spirit behind? Just interested if the order is important.
Thanks again for all the answers. I did a test extraction of I2 from Lugol's with chloroform and that worked great.
trb456
Magic Muzzlet - 25-1-2011 at 12:15
The ks3 stripper from kleanstrip is my OTC source for DCM, all I do is fill up a flask with the paste and distill, collecting all up to about 45
degrees C. The residue is easily removed afterwards. The distillate then needs to be washed with water and CaCl2 solution (saturated) then dried over
additional CaCl2. Then it is distilled for DCM of adequate purity for extractions and general solvent applications. After washings and drying it
should be free of methanol. I would not try washing it before distillation, the paste would make things very annoying, covering everything. 
The product seems to be a low percentage of MeOH so it is easiest to just distill, wash etc.
trb456 - 25-1-2011 at 13:06
Magic Muzzlet, thanks again. Two more points of clarification.
I've nevered washed or dryed before a distillation. So I know adding saturated CaCl2 draws out water, but then what? Are the missing steps: wash
with water, extract the DCM layer, add CaCl2, decant/filter, dry over CaCl2, redistill?
Second, how do you dry the solution over CaCl2, and for how long? Just put the flask in a desiccator with CaCl2 in the bottom, or is more needed?
Sorry for the rookie questions, just never something I've done.
Magic Muzzlet - 25-1-2011 at 13:36
Basically washing the DCM is having your solvent in the sep funnel, then you add water, shake etc and drain off the DCM. Then you repeat but with a
saturated sol. Of CaCl2. Then the DCM layer is removed again, and to it is added, depending on the quantity of solvent, some solid CaCl2 directly into
the flask containing the DCM. Is is shaken/stirred for a few mins then left to stand for about an hour or so. Then decant DCM into distillation flask,
distill. Just add drying agent until it stops clumping, then let it sit. I hope this answers your question 
trb456 - 25-1-2011 at 13:44
Yup, that's perfect--thanks for the patience!
trb456
SmashGlass - 26-1-2011 at 07:41
Hold the phone!
The whole experiment is supposed to show the reaction
products of the halogen gases with cyclohexane.
These are light induced (ie they go faster in the presence
of sunlight or UV irradiation).
Cl-cyclohexane bp 142 deg.C
Br-cyclohexane bp 166.2 deg.C
I-cyclohexane bp 80/82 deg.C @20mmHg
When you see @mmHg (it means a vacuum
distillation setup is required)
Using DCM (methylene chloride/ dichloromethane)
will not give the same effect as this will not react.
Now adding all 3 halogen gases at the same time to the reaction
vessle will show the relative rates of each individual reaction
by it's respective product distribution. After distillation.
The whole point of a reaction is to observe the product.
Otherwise why make it?
I have not read this experiment but I have extrapolated from
the information originally given as to what the reaction means.
If you are absolutely determined to do this reaction go to your
local high school or University, talk to a Chemistry teacher
and ask if you can try it. They might be so surprised by someone
actually interested in chemistry that they might say yes.
Obviously offer to pay for the consumables.
A university might have some analytical equipment that you
could use to see how pure you compound is too...
But a good supply of clean DCM is always handy for other experiments.
I would have suggested heptane (petroleum ether 60-80, for the old school)
for this experiment oroginally but that reaction would give mixtures of
compounds and would be too dificult to purify.
Good luck. And don't waste your money on buying paint stripper
or other supplies if it's not going to do you any good in the
first place.
trb456 - 26-1-2011 at 08:18
SmashGlass, thanks for the reply, but I don't think this is correct. I posted a link to Google Books in the initial post that will let you scroll
through the book (although maybe it does not give access to the required page). In any case, the experiment is not about the reaction with
cyclohexane. In fact, a footnote says that one could use cooking oil(!) instead of cyclohexane. The only reason for the organic solvent is
preferential extraction of the halogen. The experiment itself is about halogen-halide reactions. Specifically, extract 3 elemental halogens (Cl2,
Br2, I2), and react each with the 2 opposing halides (chosen from NaCl, KBr, KI). This is very basic stuff: I know what will happen, but have never
actually done the lab work (e.g. Cl2 will displace Br in KBr, but Br2 will not displace Cl in NaCl). Apparently I could use cooking oil, but I
figured I'd use another nonpolar solvent instead, and just wanted to make certain I was doing the right thing. The follow-on discussion about
distilling DCM was just me being schooled!
Please let me know if I'm missing something, but that's what the experiment is about.
trb456
SmashGlass - 26-1-2011 at 08:38
The link you sent didn't have the appropriate page.
They were copyright protected and I couldn't see it.
What was the halogen gasses supposed to react with?
Obviously the cyclohexan would be a very slow reaction,
but that is what it is supposed to be. It is the least polar
of the common laboratory solvents.
Many cooking oils are comporised of monounsaturated fatty
acids. Perfect for this experiment as the alkene bond C=C
would undergo halogenation much faster than the alkane
component.
If you have the glassware to make these reactions then I
would suggest you don't have to store the "haolgen gas"
in solvent but bubble it directly into the mixture.
Using petroleum ether 60/80 would be better for extraction
purposes but if you are desperate DCM would work fine for
that purpose.
Just out of curiosity. What were you planning to react the
halogens with?
Happy tinkering.
peach - 31-1-2011 at 07:37
When wondering about solvents, wiki has a good table on it with the common ones divided up into their major groups with their basic properties listed
all in one place. Scroll down until you see brightly coloured tables.
Cyclohexane, toluene and xylene all belong to the none polars.
Cyclohexane is often used as a model none polar solvent in chemistry because every bond the carbons can make has a hydrogen stuck on it and the
structure is a stable ring with no tails dangling off.
It belongs to the alkanes (the fully saturated hydrocarbons). The most simple of those is methane. And then it goes up in chain length -> methane,
ethane, butane, propane, pentane, hexane.
The first four are all gases at room temperature and pressure. Gases which usually feed your BBQ from a cylinder.
Pentane is just about long (and heavy) enough to exist as a liquid under normal conditions, but it will boil if it's warmed even slightly (36C - about
your body temperature). Because it boils and liquefies in this range, it (cyclopentane) is used as a coolant in fridges and freezers.
Hexane is the first in the series that can be realistically handled as a liquid under normal conditions. It's length also allows it to form into a
ring without stressing the bonds between each atom in the molecules. Because all of the atoms in cyclohexane are bonded to the same
number of carbons and the same number of hydrogens, it simplifies things like NMR - all of the carbons show up as one line because they're all
identically bonded. All of this simplicity in it's structure, handling and responses make it a favourite, but not always a necessity.
There are a number of occasions when no specific solvent is required, only something from within a general spectrum of them. Naphtha, from petroleum
refining, is quite often used in place of a pure none polar when people can't get the latter or don't need the purity.
One point where cyclohexane can become a problem is it's low boiling point. That makes it easy to remove from a reaction when it's done, but it also
makes it easy to remove it from a reaction before it's done. As it boils around 80C, you can't run a reaction involving it above that
temperature (a pan of water will never go over 100C whilst there is water left in it). In those instances, you'd purposefully use a higher alkane
instead.
Cyclohexane
Toluene is the same thing with a methyl (CH3) group sticking off and some double bonds
Xylene is the same again, but with two methyl groups and the double bonds. Because it has two methyls, and there are more than two places they can go
on the ring, there are isomers of it. Ortho (both groups side by side), meta (groups separated by a space) and para (groups on opposite sides of the
ring to each other)

Cyclohexane also comes in the plain stick configuration (hexane)

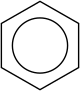
Benzene used to be a very common none polar solvent, but it has since been found to be carcinogenic and so isn't something people really want around
if there's an alternative. It also has spare double bonds, making it more reactive than cyclohexane. However, that reactivity makes it useful for
other people, and the way in which the bonds behave in the molecule is also interesting, as they appear to resonate around the ring rather than sit in
one place; which is why it has a circle in the centre of the ring instead of each double bond marked out.

[Edited on 31-1-2011 by peach]
trb456 - 31-1-2011 at 08:42
Thanks for this peach--very helpful! I did indeed fidn that WP chart, and it was the basis for my initial conclusion that toluene might be a good
substitute. Further reading suggested that it was the very low reactivity of cyclohexane that was the reason for its choice of extracting highly
reactive halogens. Other solvents will clearly work: I did a test I2 extraction with CHCl3 and it worked fine. But based on some other comments, I
did finally find a source for a small amount of cyclohexane, so I'll wait to do the experiment as presented. Thanks for all the help!
trb456
ThatchemistKid - 5-2-2011 at 13:28
Sorry for the late reply. The reason that I said distill it away from the paste first before washing and re-distilling is that the paste mixture is
thick and goopy, trying to wash that just ends up in a horrendous mess, but distilling away the dichloromethane from it is straightforward.










