
Mildronate - 18-9-2010 at 06:59
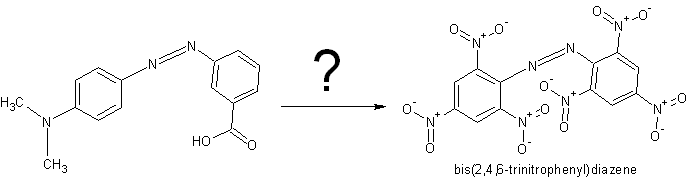
Is it possible to make bis(2,4,6-trinitrophenyl)diazene from methyl red or some else explosive from it?
497 - 18-9-2010 at 07:51
Doubt it. Getting that carboxylic acid and dimethylamine off would be a pain in the ass, and I doubt it could be done without destroying the azo
linkage anyway. Just nitrate aniline to trinitroaniline and then form the azo compound from that.. Much easier I suspect.
Mildronate - 18-9-2010 at 08:08
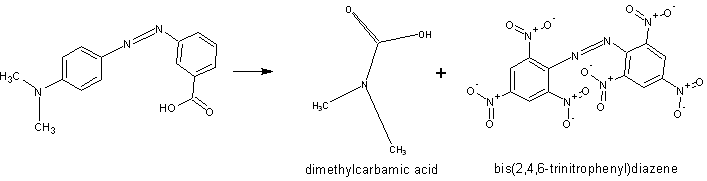
i had many botlles of methyl red. Maybe is possible this way?
[Edited on 18-9-2010 by Mildronate]
[Edited on 18-9-2010 by Mildronate]
497 - 18-9-2010 at 14:38
Any references for that? I really doubt those C-C and N-C bonds are going to break the way you want.
hissingnoise - 19-9-2010 at 06:56
Bis(2-4-6 trinitrophenyl)diazene (aka hexanitroazobenzene (HNAB)) is prepared by reacting dinitrochlorobenzene and hydrazine and nitrating and
oxidising the tetranitrohydrazo product by mixed acid. . .
You can forget methyl red!
Mildronate - 19-9-2010 at 07:13
Why forget structure is very similar.
hissingnoise - 19-9-2010 at 08:57
Why waste time grasping at straws - as 497 said, removing the side-chains while keeping the nucleus intact would probably be quite a feat. . .
And nitrating azobenzene itself is likely to be anything but straightforward!
