...a theoretical synthesis to this compound from easily available chemicals. not for illicit purposes, simply because of the chalange and enjoyment of reaching the theoroetical goal.
...anything producing a reasonable amount will be adiquete.
...if there is a hurdle that maybe a little to hard to jump in this synthesis that can be overcome by changing the end product into something similar with equal or greater value...





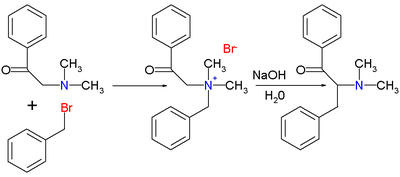
 +
+  +
+  =======>
=======> 
 +
+ 
 .references if requested
.references if requested