
franklyn - 30-6-2009 at 14:37
From Overviews of Recent Research on Energetic Materials
www.tinyurl.com/adwu4v ,
Previously posted here _
http://www.sciencemadness.org/talk/viewthread.php?tid=7518&a...
- Bottom of page 474
" It is commonly accepted among theoreticians that the upper limit of density
for compounds constituted of the first-row elements is approximately 2.2 gm/cc. "
- Top of page 475
" The most intriguing exception to this limiting theory is the case of diamond,
which is not a true crystal lattice, but rather, a covalently bonded, three
dimensional polymer of elemental carbon; its density is approximately 3.5 gm/cc.
Clearly, if one were able to construct an oxidatively balanced, three dimensional,
covalent network of energetic functional groups, then densities far in excess
of 2.2 gm/cc could be realized. "
- Middle of page 499
" The alleged constraint that the density of ensembles of the first-row
elements cannot exceed 2.2 gm/cc may well be artificial and erroneous given
that diamond has a density of 3.5 gm/cc. While it is idealistic to maintain
that this can be readily attained in energetic materials, it is reasonable to
assume that certain carbon/nitrogen polymers can have densities exceeding
2.5 gm/cc without compromising enthalpy. "
My note :
- I agree, and while the premise advanced by me in this particular post is speculative - it is only a difficult challenge not an impossibility.
__________________________________
Cyanamide exists as two isomers ,
Amidocyanogen H2N-CΞN , and Carbodi-imide H-N=C=N-H
( These slowly polymerize into dicyandiamide dimer, and when that is heated over 150 ºC
it vigorously further polymerizes into the trimer tricyantriamide and it's tautomer melamine )
I propose chlorinating Calcium cyanamide to yield Carbo-di-chloramine ,
( note that the trimer hexachloromelamine is a known compound )
Ca=NCN + 2 Cl2 -> CaCl2 + ( ClN=C=NCl )
- C L I C K thumbnail to enlarge -

which then is reacted by the known nucleophilic substitution between chloramines and
metal azides , with Sodium Azide to form Carbo-di-azidamide _
ClN=C=NCl + 2 NaN3 -> 2 NaCl + (N3)N=C=N(N3)
- C L I C K thumbnail to enlarge -
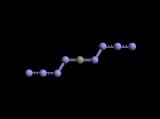
The prospect of two four nitrogen chains dangling off a lone carbon implies to me
a more stable two ring structural form depicted below is a more likely result _
- C L I C K thumbnail to enlarge -

Internal long range structure cannot be the sole consideration for a viable
monomer moiety since the surfaces must also be self consistent and cannot be
made of free radicals.
The task then is inducing adjoining polymerization which forms naturally into a
tetrahedrally connected lattice. The simplest repeatable cell of this diamondoid
lattice of Poly-tetrazomethane ( below left ) with carbons separated by 4 nitrogens
is highlighted yellow in this depiction below center _
- C L I C K thumbnail to enlarge - . - C L I C K thumbnail to enlarge -
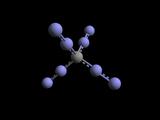


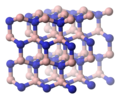
a form exemplified by adamantane shown in the middle above here _|_ overall
structure is viewed more clearly in the depiction of Boron Nitride above right.
Evidence that this should be favored to occur comes from the observation that
opening a ring bond facilitates the formation of two bonds, although overall the
total number of bonds remains constant, the conversion to less strained straight
line bonds is exothermic, making it an inherently preferential outcome. Militating
against coupling is the presence of lone electron pairs at the unbound azo
extremities, unless mutual resonance is synchronized with formal charges in
opposite phase.
Validating the premise forwarded by the author in the book cited above.
Overviews of Recent Research on Energetic Materials
Projected performance is estimated applying the method discussed here _
http://www.sciencemadness.org/talk/viewthread.php?tid=11195&...
Corrected calculation for
http://www.sciencemadness.org/talk/viewthread.php?tid=11195&...
- The arithmetic is detailed in the text file attached below , also a zip containg the Arguslab files -
In determining density, the factor K is entirely omitted or else assigned a value
of one, since this is macroscopically a single molecule rather than a conglomeration.
Thus using the molar weight in full for calculation the value of density is ρ = 2.59 gm / cc
In determining heat of explosion, the sum of average bond energies is compared
to those of the detonation products. The estimated explosion energy is 1940 Kcal / Kg
while this may seem high, it is within the bounds of energy of known high nitrogen
endothermic compounds and groups. For example Diazomethane with only one azo group
is CH2=N=N +63.1 Kcal/mol , + 1500 Kcal / Kg. made up of 66 % nitrogen. Proportional to
the percentage of nitrogen, at 90 % a tetra azo must have energy at least a third higher.
Note also that 3,6-Diazido-1,2,4,5-tetrazine
http://www.sciencemadness.org/talk/viewthread.php?tid=9370#p...
works out to + 1585 Kcal / Kg at 85 % nitrogen , but with a less energetic tetrazine group
Detonation Pressure is an astounding ~ 779 kilobars
Projected Velocity of Detonation ~ 12,000 meters / sec
Due to the large network spacing , the material assumes the caged formation
of a zeolite. This raises the possibility of being able to fill the hollow voids by
gasing under pressure with an additional admixture of energetic molecules,
raising the density and augmenting the energy. A likely candidate would be
nitrous oxide with a heat of formation of + 19.5 Kcal / mol , + 443 Kcal / Kg
Reasonably surmising that a one to one ratio of carbon to nitrous oxide is
achievable, since the volume does not change , by adding N2O , the density
increases markedly to 3.473 gm / cc
The estimated explosion energy of the interstitial compound is 3395 Kcal / Kg
Detonation Pressure is an unreal ~ 1900 kilobars
Projected Velocity of Detonation is equally fantastic at ~ 17900 meters / sec
__________________
A property worth mentioning is that a lattice comprised of resonant or alternating
single and double bonds will be electrically conductive. This will make the material
immune to initiation from ordinary static charges as this will be drained away. It also
raises the interesting possibility of applying this property to electrical initiation relying
on the joule heating as is done with exploding bridgewires.
The initial presence of a magnetic pole pointing coaxially in the direction of detonation
or alternatively the circumferential Biot - Savart magnetism of a longitudinal current ,
will induce a current to run radially from the column in the reaction zone of the detonation
wave. If the column is wrapped with an insulating film and sheathed in a conducting foil
connected to the column at the far end , because of the resulting coaxial current , a self
generated electrical pulse will result much as from a magnetic flux compression generator.
What interesting det cord that would make.
- It has not escaped my attention that the very same thing can be done with any
explosive if it is substituted for the dielectric insulator in coaxial cable. Otherwise known
as a Blumlein capacitor.
__________________________________________
Some related articles
Synthesis of Carbon Nitride C3N4 ( not yet realized )
http://handle.dtic.mil/100.2/ADA359222
Syntheses and Structures of Metal Cyanamide Compounds
http://deposit.ddb.de/cgi-bin/dokserv?idn=966164989&dok_...
Diazomethane , Azimethylene , Diazirine
CH2=N=N ( +63.1 Kcal/mol )
http://en.wikipedia.org/wiki/Diazomethane
http://sites.google.com/site/archibaldchemistry/Home/diazome...
http://sites.google.com/site/archibaldchemistry/Home/chemist...
http://sites.google.com/site/archibaldchemistry/Home/chemist...
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV5...
Hydrazoic Acid paper
http://sites.google.com/site/archibaldchemistry/Home/chemist...
Post on Tetraazidomethane
http://www.sciencemadness.org/talk/viewthread.php?tid=8271&a...
Related posts
N - Azidamines
http://www.sciencemadness.org/talk/viewthread.php?tid=9370
Performance & density
http://www.sciencemadness.org/talk/viewthread.php?tid=11195
Sources of Bond Energy citations
http://www.cem.msu.edu/~reusch/OrgPage/bndenrgy.htm * *
http://www.wiredchemist.com/chemistry/data/bond_energies_len...
.
Attachment: Poly-tetrazomethane.rtf (25kB)
This file has been downloaded 1390 times
Attachment: Poly-tetrazo methane.zip (154kB)
This file has been downloaded 666 times
PHILOU Zrealone - 15-7-2009 at 10:38
Very good writing!
Stil just to point this out:
Density of some (ionic and covalent) compounds made out of first row elements (actually they speak of the second row) are over 2,2 g/ccm...
d LiF anhydrous = 2,635
d LiNO3 anhydrous= 2,380
d LiOH anhydrous = 2,540 (although the monhydrate LiOH.H2O = 1,510)
d Boron cristaline = 2,350
d B2O3 = 2,460
d Boron nitride hexagonal modification (layer structure similar to graphite) = 2,290 (vs graphite 2,255)
d Boron nitride cubic modification (borazon diamond structure like) = 3,487 (vs diamond 3,50-3,55)
But this doesn't change much the theorical point of your writing  because it
enhances the possibilities.
because it
enhances the possibilities.
I'm glad that you also think, like I do, that higher polymeric structures displays better performances than lower polymers (dimers, trimers,
tetramers,...) or than monomers.
"Great minds always encounters" (as we say in french).
************************
For the rest because you like links 
1a)
quoted from: http://www.rsc.org/Publishing/Journals/FT/article.asp?doi=FT...
"conductivity measurements show that pristine poly(CNCN) and poly(NCCN) are an insulator and a semiconductor, respectively."
1b)The following table out of: http://www.whxb.pku.edu.cn/EN/article/showCorrelativeArticle...

So supramolecular double bonding or aromaticity (sp2) resonance doesn't imply, per se, good conducting abilities especially in the case of CN
binary compounds, but it might imply supraconductivity abilities.
2)
quoted from: http://www.mrs.org/s_mrs/sec_subscribe.asp?CID=12305&DID...
"The composition and properties of the sp 2-bonded carbon nitride precursor paracyanogen (pCN) has been studied at high pressures and temperatures.
Paracyanogen decomposes to carbon and molecular nitrogen with the decomposition temperature increasing with pressure over the range of 3 to 19 GPa
(Pressure annealing to 17 GPa yields a solid with a density of 2.1 g/cm). Prior to decomposition, pCN can be transformed to an atmosphericpressure
quenchable phase that is more than 25% higher in density and over an order of magnitude harder than the starting material. Structural analysis of this
quenchable phase shows, however, that it consists of a sp2-bonded network. In addition, the decomposition kinetics of paracyanogen have been studied
in detail. Rapid, self-propagating decomposition occurs above a threshold temperature. Below this, decomposition rates exhibit an Arrhenius behavior
with activation energy and volume of 2.7 eV and 3.9 A3, respectively The decomposition rates depend on the nitrogen density and decrease significantly
with lower nitrogen concentration. Kinetic effects favoring a graphite-like, sp 2-bonded structure may preclude the high-pressure synthesis of
superhard, sp 3-bonded carbon nitride solids below their thermodynamic stability limit, unless an optimally designed precursor is employed."
Note the 2,1 g/ccm density  !
!
Also N#C-C#N is endothermic from its elements and decompose explosively from a spark, the polymer is very stable and has a high activation energy but
above a high enough T° the decomposition is fast (but I don't think it would be explosive
3)
Out of Polymeric Materials Encyclopedia (p 4885-4888): http://books.google.be/books?id=LPpfUGaBsHgC&pg=PA4887&a...
This book explains that the structure of paracyanogen initially thought as ladder type is actually open distorded what could explain the
conductivity not being of the metallic type but increasing with temperature and field.
4)
Out of: http://scitation.aip.org/getabs/servlet/GetabsServlet?prog=n...
"The density, the hardness, and the internal stress of the films present a similar dependence on the annealing temperature, i.e., they increase with
the temperature of the thermal treatment. The thermal treatment induces a structural modification on the carbon–nitrogen films changing from a soft
paracyanogen-like material to a harder and more graphitic one"
So the ladder type of polymer (graphite structure type) might be the explanation to the density increase
5)
Out of: http://www.sciencedirect.com/science?_ob=ArticleURL&_udi...
"On the other hand, these nitrogen-rich films turned out to be mainly sp2 bonded having rather low densities of 1.8–2.0 g cm³ only, irrespective of
the method. "
1,8-2,0 g/ccm is already very good I think for a CN polymer
6)
Out of: ftp://ftp2.biblioteca.cbpf.br/pub/apub/1998/nf/nf_zip/nf0359...
They mentions a reduction of N/C ratio from 0,71 to 0,52 by thermal annealing on amorphous carbon nitride films and an increase of density
from 0,8*10E23 atoms/ccm to 1,2*10E23 atoms/ccm.
If I calculate correctly this means an increase of density from:
(0,8/6)*(0,71*14+0,29*12)= 1,789 g/ccm
to
(1,2/6)*(0,52*14+0,48*12)= 2,608 g/ccm
(1 mole of atoms being 6*10E23 and the Atomic Mass of nitrogen being taken as 14 and that of carbon being taken as 12)
As conclusions:
1)Polymerisation of your 3N-N=C=N-N3 might be tricky and lead to non-perfect 3D polymers (under very high pressure and maybe T°) what could reduce a
bit your target density!
2)It can rearrange to (3N)2N-C#N and than trimerise to a melamine sturcture and further polymerise to a planar structure with polyaza bridges ...this
not being detrimental to your theroy 
3)During polymerisation N2 moieties might be split of out of your tetraaza bridges reducing them to diaza bridges, but if detrimental to the structure
and energy of your intitial product,the network will be denser.
4)A lot of aspects mentionned in the above links give higher densities than expected, and the stability of polymers is higher than monomers what migth
be very good and in the sense of the feasability of your target molecule 
So only time and experiment will tell the truth about your theory
[Edited on 16-7-2009 by PHILOU Zrealone]
497 - 2-10-2009 at 19:41
You might have trouble even getting your carbodichloramine.. According to this halogenation of carbodiimide yields only dichlorocyanamide. Though dichlorocyanamide may have some interesting uses of its own.
A rose by any other name
franklyn - 4-10-2009 at 11:43
Thanks for that reference
Chemistry & Technology of Carbodiimides Chap 4 - Halogenated Carbodiimides , 4.1 introducton
Yes I see that _
Carbodiimides with halogenated substituents on the nitrogen atoms of the cumulative double bonds are not known.
Apparently, dihalocyanamides are formed in attempts to synthesize N,N'-dilhalocarbodiimides. An example is the
fluorination of cyanamide. which produces exclusively the highly explosive difluorocyanamide.1 Theoretical calculations
on the hypothetical FN=C=NF have been published.2. (- My note - references given unavailable from book preview )
My conjecture _
Cyanamide exists as two isomers ,
Amidocyanogen H2N-CΞN , and Carbodi-imide H-N=C=N-H
Analogously Dichlorocyanamide can be
Dichloroamidocyanogen Cl2N-CΞN , and Carbodichlorimide ClN=C=NCl
Ambient conditions , temperature , even solvation has to matter.
" dichlorocyanamide may have some interesting uses of its own "
Not as high value , Hexachloromelamine for example is known.
.
AndersHoveland - 27-6-2011 at 13:37
very interesting, but not sure if it would actually work
as much as I understand, the CN8
[-]N=N[+]=N--N=C=N--N=N[+]=N[-]
would immediately decompose.
the first intermediate would be
[-]N=N[+]=N--N=C=N[+]=N[-] NΞN
Likely it would decompose to cyanogen azide, NΞC--N3, and nitrogen gas, if not an explosion
Perhaps tetrazidomethane, C(N3)4, could be induced to polymerize. Chains of four nitrogen atoms without any hydrogen are probably not stable, but
perhaps the nitrogens could take a more lattice-like structure, with fewer double bonds. something like this may be possible under pressure or if kept
at liquid nitrogen temperatures
R3C--N--N=N--N--CR3
.............l...............l
R3C--N--N=N--N--CR3
several solid allotropes of elemental nitrogen are known to exist: https://sites.google.com/site/ecpreparation/pentazenium-pent...
[Edited on 27-6-2011 by AndersHoveland]
IndependentBoffin - 27-6-2011 at 23:36
Please excuse me wading into this topic as an aerospace engineer and not a chemist  but AIUI diamond has such a high density because it is basically a 3D macromolecule where most carbon atoms that constitute it are covalently
bonded to other carbon atoms.
but AIUI diamond has such a high density because it is basically a 3D macromolecule where most carbon atoms that constitute it are covalently
bonded to other carbon atoms.
Logically, if one wants to produce energetics with a high density you will have to:
1) Get rid of hydrogen atoms (mostly empty space)
2) More importantly, produce a compound with very strong inter-molecular bonding. In diamond's case, its inter-atomic bonding is effectively
intra-atromic covalent bonds, hence its strength, short bond lengths and high density.
Weaker intermolecular bonding -> long bond lengths -> low density.
Unless you can create a 3D CN macropolymer with everything covalently bonded to each other, rather than linear molecules with weak (polar?) bonding
between them, its density will always be less than diamond.
Please correct my understanding if it is wrong 







 because it
enhances the possibilities.
because it
enhances the possibilities.
 !
!


 but AIUI diamond has such a high density because it is basically a 3D macromolecule where most carbon atoms that constitute it are covalently
bonded to other carbon atoms.
but AIUI diamond has such a high density because it is basically a 3D macromolecule where most carbon atoms that constitute it are covalently
bonded to other carbon atoms. 