
Krypton - 16-12-2003 at 05:20
To a reflux condenser add 200 ml 96% H2SO4 and 20 ml, 85 % HNO3 and 10 g of trichlorobenzene and reflux for 4 hours at 145C and than cool , add 1 l
cool destilled water and filter out the crystals and dry at room temperature.
Stirr the mixture with a magnetic stirrer over the time and do not overheat the mixture. Control with a thermometer.

[Edited on 16-12-2003 by Krypton]
[Edited on 16-12-2003 by Krypton]
BASF - 22-12-2003 at 03:51
What are the yields of this reaction?? - Three chlorines on the benzene ring > highly deactivated ring.
Isnt the product mainly the mono- or dinitro- derivate ?...
However, the reaction conditions seem to be of the "brute force" kind(4 hours reflux at 145C)
What is the source?
[Edited on 22-12-2003 by BASF]
Chris The Great - 15-3-2005 at 20:03
While trying to find info on making 1,3,5-trichlorobenzene, I stumbled into this thead, and since I have some info about this compound right now....
From Chemistry and Technology of Explosives, Volume I, page 469
Manufacture of 1,3,5-trichloro-2,4,6-trinitrobenzene. The I. G. Griesheim Works applied the following process for the manufacture of
the above product.
The reactor is charged with 980 kg of 30% oleum to which 100kg of solid trichlorobenzene is added during the course of one hour. The whole is heated
to 100*C for another hour and kept at this temperature for 2-3 hr longer with constant stirring. Towards the end of this operation sulphonation takes
place. The reaction may be considered complete when a sample of the mixture taken from the nitrator dissolves completely in water.
After cooling the reactor contents to 50*C 300kg of 99% nitric acid are added during about 4 hr. While this is being done the temperature rises to
100*C. After all the nitric acid has been added, the mixture is stirred for another 10-14 hr, then during the course of a further 8 hr the
temperature is gradually raised to 140-145*C. As too rapid a rise in temperature would involve the risk of decomposition, if this occurs the nitrator
contents should be drained off into a safety tank. After a temperature of 140-145*C has been attained, the mixture in the nitrator is stirred at this
temperature for a further 45 hr. At this stage of the process the product of the reaction crystallizes. Then the nitrator contents are cooled down
to 20*C and transferred by compressed air to the filter. The filtered product is transferred to a washing tank, where it is washed by mixing with
cold water, followed by decantation, several times until free of acid (Congo paper test).
The spent acid contains 13% of HNO3 and H2SO4 + SO3 equivalent to 90% H2SO4.
The washed, acid-free product is filtered off on a vacuum filter and dried under reduced pressure at 100*C. In this way 125kg of the product melting
at 189-190*C are obtained, which corresponds to 72% of the theoretical yield.
franklyn - 1-6-2010 at 23:12
Certainly not something to be tried up close and personal , and you'll probably think
I'm crazy for even proposing it. Since the only requirement is stirring and temperature
control which can be monitored remotely, a reaction vessel placed in a pit outdoors
provides the necessary precaution.
Hexachlorobenzene which had seen use as a fungicidal fumigant , now banned as a
persistent enviornmental pollutant, limits it's availability but can readily be made.
It has a melting point of 227 - 231 ºC , and boils at over 323 ºC.
Sodium nitrite ( NaNO2 ) melts at 271 ºC decomposing above 320 ºC.
The gist is this , according to the following stoichiometric balanced equation
C6Cl6 + 6 NaNO2 => 6 NaCl + C6(NO2)6
a melt of the two materials ( with some Aluminum Chloride ( AlCl3 ) lewis acid catalyst )
might directly yield Hexanitrobenzene which itself has a melting point of 256 - 264 ºC
with the NaCl salt settling out.
Readily hydrolyzed by water into 2,4,6-trinitrophloroglucinol , the anhydrous conditions
of the melt will conserve it.
1,3,5-trichloro-2,4,6-trinitrobenzene might be the only yield , with a lower melting point
of 190 ºC yet remains thermally stable up to 290 ºC only decomposing above 350 ºC.
Clearly no one has proposed melting that with NaNO2 to produce Hexanitrobenzene,
so this may be as far as it can go.
http://en.wikipedia.org/wiki/Sodium_nitrite
Process for production of Hexachlorobenzene US2792434
http://en.wikipedia.org/wiki/Hexachlorobenzene
http://cameochemicals.noaa.gov/chemical/3556
http://www.lookchem.com/HEXACHLOROBENZENE
 http://en.wikipedia.org/wiki/Hexanitrobenzene
http://en.wikipedia.org/wiki/Hexanitrobenzene
Trade Names and Synonyms:
Hexa CB
HCB
Phenyl perchloryl
Perchlorobenzene
Pentachlorophenyl chloride
Anticarie
Bunt-cure
Co-op hexa
Julin's carbon chloride
No bunt 40
No bunt 80
Sanocide
Snieciotox
Smut-go
Granox nm
Voronit C
Other names:
Amatin
Bunt-no-more
No Bunt Liquid
Julen's carbon chloride
Hexa c.b.
Hexachlorbenzol
Ceku C.B.
Esaclorobenzene
Saatbeizfungizid
Julian's carbon chloride
Julin's chloride
Hexcachlorbenzen
.
franklyn - 3-6-2010 at 19:02
To late to edit , just to mention anhydrous Aluminum Chloride
melts at 192 ~ 193 ºC but actually begins to sublime at 180.
It is soluble in organic chlorides. Some kind of reaction must
result, the question is will that be an explosion.
.
franklyn - 16-7-2010 at 20:31
This post shows the merits of ab initio calculation in appraising
a prospective energetic compound.
According to an excerpt from Nitrocarbons - Arnold T. Nielsen
http://search.barnesandnoble.com/Nitrocarbons/Arnold-T-Niels...
the hypothetical bicyclic nitrocarbon , Octanitronaphthalene has not
been isolated. It would be expected to be less hydrolytically liable
than Hexanitrobenzene. While initially seeming to have much promise
calculation shows that moderated performance is the result of the
negative oxygen balance which reduces the energy output by
260 Kcal / kg or 16 % compared to Hexanitrobenzene - 1633 Kcal / Kg
348 g/mol
C6N6O12 => 6 CO2 + 4 N2
+ 4.2 . . . . . . . 6 (- 94) . . . (- 568.2 / 348 ) x 1000 = - 1633 Kcal / kg
Octanitronaphthalene
. . . . . . . . . . . . . . . . . . . . . . . . . . . 488 g/mol
C10H8 + 4 N2O4 => 4 H2 + C10 N8 O16 => 6 CO2 + 4 CO + 4 N2
+ 18.8 . . 4 (- 4.7) . . . . . 0 . . . . . . . 0 .
. . . . . . . .6(- 94) . 4(- 26.4)
(6 CO2 is - 564) + (4 CO is - 105.6) = - 669.6
(- 669.6 / 488 ) X 1000 = - 1372 Kcal / kg
Estmated Heat of formation is 0 , which corresponds with the
slightly endothermic Hexanitrobenzene. The heat of explosion
then is just the combustion of carbon , - 669.6 Kcal /mol
An empirical method to estimate detonation velocity described
on page 6 here -> Explosive Properties of Polynitroaromatics
http://handle.dtic.mil/100.2/ADA229627 , gives a value of 4.98
for factor F which works out to 8580 m/s. The utility created by
forum member engager , utilizing the methodology of Keshavarz
obtains practically the same value ( 8500 ) at a nominal density
of 1.8. The method I explained in these other posts
http://www.sciencemadness.org/talk/viewthread.php?tid=11195#...
http://www.sciencemadness.org/talk/viewthread.php?tid=11195&...
is in agreement with detonation velocity of 8550 and 340 Kbar
pressure at the projected density of 1.96.
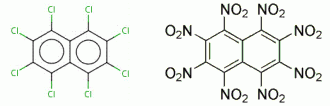
As I speculated in the preceding post above , perchlorinated Napthalene
might similarly serve as a precursor for Octanitronaphthalene. A possible
approach to synthesis of Perchloronaphthalene would be a heated admixture
of Naphthalene with Trichloroisocyanuric acid , possibly catalysed , to
precipitate Cyanuric acid in neat Octachloronaphthalene. The various
known methods of naphthalene chlorination are referenced below.
Octachloronaphthalene
SYNONYMS:
Perchloronaphthalene
Halowax
Halowax 1051 ( is 10 % hepta & 90 % octa )
CAS: 2234-13-1
EINECS 218-778-7
IMIS: 1955
NIOSH: RTECS QK0250000
Pale yellow waxy solid with an aromatic odor.
Molecular Formula C10Cl8
Molecular Weight 403.73
Melting point 192 °C
Boiling point 440 ºC
Vapor Pressure: <1 mm Hg at 20 °C
Density 2.00
Soluble: Benzene , Carbon Tetrachloride
Water solubility: Insoluble
Incompatible: Strong oxidizers
Photolytic decomposition
Uses : Dielectric Insulator , Fire proofing
Exposure Routes
inhalation, skin absorption, ingestion, skin and/or eye contact
Symptoms
Chloracne-form dermatitis; liver damage, jaundice
Bioactivity similar to Dioxins
Polychlorinated Napthalenes
http://www.nicnas.gov.au/Publications/CAR/Other/S48_PCN_July...
Polychlorinated Napthalenes
http://www.unece.org/env/lrtap/TaskForce/popsxg/2000-2003/pc...
Chlorinated Napthalenes
http://whqlibdoc.who.int/publications/2001/9241530340.pdf
" Electrophilic Aromatic Chlorinations carried out with Chlorine itself
and a suitable catalyst such as Ferric Chloride "
G. Olah , A. Pavlath , G. Varsanyi - J. Chem Soc., 1957 , 1823
" Perchlorination of polycyclic Aromatic Hydrocarbons "
M. Ballester , C. Molinet , J. Casteñer - J. Am Chem. Soc., 1960 , 82 , 4254
Aromatic Substitution. XVII.1
Ferric Chloride and Aluminum Chloride Catalyzed Chlorination of Benzene,
Alkylbenzenes, and Halobenzenes
G. Olah , S. Kuhn , B. Hardie - J. Am. Chem. Soc., 1964 , 86 (6) , 1055–1060
" Perfluorinated Aromatic Compounds "
Frazer & wallenberger - J. Polymer Science , Part A , 1964 , 2 , 1147
" Chlorinations with Chlorine gas carried out in the solid phase "
J. of Org. Chem., 1974 , 1744
.
franklyn - 19-7-2010 at 22:11
Nitration of Benzene or Toluene in a Molten Nitrate Salt - US patent 4804792
http://www.google.com/patents/download/4804792_Nitration_of_...
World Patent
http://tinyurl.com/22ne7zw
.


 http://en.wikipedia.org/wiki/Hexanitrobenzene
http://en.wikipedia.org/wiki/Hexanitrobenzene