Silver Perbromate. Silver oxide (5.80 g, 0.025 mol) was added in portions with stirring, to 100 ml of 0.5 M perbromic acid. The
mixture was stirred for 2 hr at ambient temperature and was then filtered. The bulk of the water was removed from the filtrate under vacuum, and 100
ml of benzene was added. The remaining water was removed by azeotropic distillation using a Dean-Stark trap. The resulting benzene solution was
filtrered hot. When the solution was cooled to room temperature, 100 ml of hexane was added. The solvent was decanted from the precipitated salt,
and the salt was dried briefly at 25C (0.05 mm) and was then heated with a 70C bath at 0.05 mm for 6 hr to remove absorbed solvent. The product, 11.1
g (88%), was a white, hygroscopic, crystaline solid.
.............
Isopropyl Perbromate. A solution of 0.123 g (1.0 mmol) of isopropyl bromide in 1 ml of carbon tetrachloride was added dropwise, with
stirring, to a suspension of 0.252 g (1.0 mmol) of silver perbromate in 4 ml carbon tetrachloride, maintained at -20C by means of a carbon
tetrachloride - Dry ice slush bath. The reaction mixture was kept at -20C for 15 min. The silver bromide was removed by filtration, giving a pale
yellow solution.
.............
The yield of isopropyl perbromate was 95% determined by nmr integration using chlorobenzene as a quantitative internal standard. The only impurity
detected was 1% of acetone. The decomposition of isopropyl perbromate solutions was monitored similarly by nmr. The solutions showed no
decomposition within several hours at -20C. At ambient temperature, the compound decomposed with a half-life of about 30 min to give acetone as the
only product detectable by nmr. The yield of acetone was 90% in 24 hr. The solution became red-orange in color. |












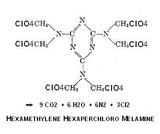

 franklyn seems to
be using it as a formaldehyde source in the scheme he outlined in his post.
franklyn seems to
be using it as a formaldehyde source in the scheme he outlined in his post.
 I don't want to think what
that would do with powdered calcium metal...
I don't want to think what
that would do with powdered calcium metal...  ^2
^2
 I am already starting the preparations
of fixing blast screens, getting protective gear, installing a fume hood of sorts, etc. I am, after all, going all out on this, and wish to maybe
improvise it if the school reneges on the deal.
I am already starting the preparations
of fixing blast screens, getting protective gear, installing a fume hood of sorts, etc. I am, after all, going all out on this, and wish to maybe
improvise it if the school reneges on the deal. 