
indigofuzzy - 13-5-2008 at 22:36
I was just wondering what thoughts all of you here might have on alternative periodic tables. I've seen a couple (spirals, 3d billboard-like
constructions, hexagonal arrangements, etc)
I wonder also what are some thoughts on the "standard" periodic table. (its plusses and minuses)
I ask all of this because I've been working on an alternative table for purely artistic reasons, that is, I'm making one for its aesthetic value, and
not because i have any delusions of making a "better" table, just a prettier one.
here's my rough draft:

I'm calling this draft a "periodic spiderweb"
The final draft will have nice curves instead of jagged lines, and include info to actually make it meaningful. (atomic number, atomic weight,
labeling the periods, a key to the color code, etc.)
Well, it's just a rough draft, but any thoughts or suggestions?
YT2095 - 14-5-2008 at 00:14
Very Nice!
I am a little Curious as to Why H connects with F though?
the He and Li make sense, but not the F, or am I missing something?
not_important - 14-5-2008 at 00:32
H can lose an electron like Li to give h(+), or gain one like fluorine to for H(-) as in LiH (but not the covalent hydrogen hydride)
To me, the H to He link doesn't make sense, as hydrogens valence shell is not filled.
JohnWW - 14-5-2008 at 00:53
That periodic table should be extended beyond element 118 (an inert gas), up to element 126, which would have at least some 5g instead of 6f
electrons (which could mean that it may not be octovalent like Pu is), because of the theoretical prediction that an isotope of it with a suitable
number of neutrons, with an atomic weight well over 300, may be stable or very long-lived. Some mostly Israeli scientists recently reported the
discovery of small amounts of a very long-lived isotope of element 122 in thorium.
YT2095 - 14-5-2008 at 00:53
well the way I`m looking at it, on my PTOE, the H is directly above Li, and across from He.
although I have seen some where H is just "Floating" in a box of it`s own.
as for the actual Design I think it looks great, it reminds me of the one I did out of paper the once, top down it looked like a figure `8`.
if it was made into a Poster, I`d be happy to buy one 
woelen - 14-5-2008 at 01:22
Particularly nice is the lanthanoids and actinoids. These nicely are grouped and it is clear at once that they are "extensions" of the transition
metals and that for Sc at lower rows there is a whole group of rather similar elements.
The only point with this is that an element like uranium, which has quite similar chemistry, compared to chromium, molybdenum and tungsten, now is
connected to neodymium. Neptunium more obviously is more similar to vanadium et al. But these are not real flaws of the drawing, it is more a
descriptive similarity, than one truly based on the periodic table. In older texts, these elements indeed were grouped like (Cr, Mo, W, U) as being a
column of metals with related chemistry.
indigofuzzy - 14-5-2008 at 02:11
Well, I could draw one up tomorrow and include at least one period with the g-blocks.
Who knows though, when the elements above 118 are found and studied, exactly how their electrons would configure themselves. Sometimes, Mother Nature
has surprises for us.
indigofuzzy - 14-5-2008 at 03:39
Ok, added the 8th and 9th periods, including the g-Blocks. Here's the new rough draft:

Oh, the image is kinda big. I can scale it down if needed... I made it on a 30" monitor, so I've kind of lost perspective on how much area a "normal"
monitor has...
Edit: The picture has been scaled down to 700 pixels wide. To see the original, click here.
[Edited on 5.14.2008 by indigofuzzy]
12AX7 - 14-5-2008 at 09:12
I've got a 19" monitor and it's too big. I keep my browser window about 800 wide. Images should never be over 700 pixels wide... if you can't say it
in 700 pixels, try again.
Quantum numbers obfuscate any effort to produce a continuously curved mesh. The tight curvature of H connecting with Li, He and F is quickly
contrasted by the dramatic widening caused by the d block (look how long the Be-B and Mg-Al edges are!), to say nothing of the f and g blocks. An
intuitive structure of the periodic table may not be possible (quantum isn't intuitive), but a piecewise geometric structure could still be made based
on the ratios of elements in a row. Putting that into an elegant, possibly curved structure, Idunno, you're the artist, I'm just the critic. 
Incidentially, shouldn't Sc and Y be orange, and if Y is linked to the lanthanides, shouldn't Ac be linked to the g block top row and Al to the d
block top row likewise?
Tim
indigofuzzy - 14-5-2008 at 15:30
I connected Sc and Y to the lanthanoids and actinoids because of having read that the properties of Sc and Y were very similar to the properties of
the Lanthanoids / Actinoids. I was also basing this on what my 8th grade science teacher said (over 12 years ago) that "the lanthanides[sic] and
actinides[sic] are so similar that they almost belong in the same square. Well, and there was also Oliver Sachs, who wrote in "Uncle Tungsten" about
the f-Block elements being so similar that even Nature had trouble telling them apart.
So, maybe I should have connected all of the g-Block metals to Actinium. It certainly would add to the aesthetic continuity.
I actually had thought of connecting aluminum to the d-Block, but it was kind of a toss-up between aluminum and magnesium, with some uncertainty as to
which one is most similar to the transition metals.
But, since I'm not an expert on any of this, connecting Sc and Y to the f-Block may have been a big goof.  Then again, I could say the same about having connected Hydrogen to any column at all
Then again, I could say the same about having connected Hydrogen to any column at all 
12AX7 - 14-5-2008 at 22:01
Definitely, aluminum would fan out to the d-block. After all, Mg, Sr and Ra are much more similar than Al, Y and Ac.
Oh- the matter of connecting hydrogen: that should be with He, since they form a single row of two. This row connects in the standard manner to the
next row (so H would connect to Li through F below it, and He to Ne below it), or something to that effect.
Tim
P.S. That's better, but the first picture is still huge. 
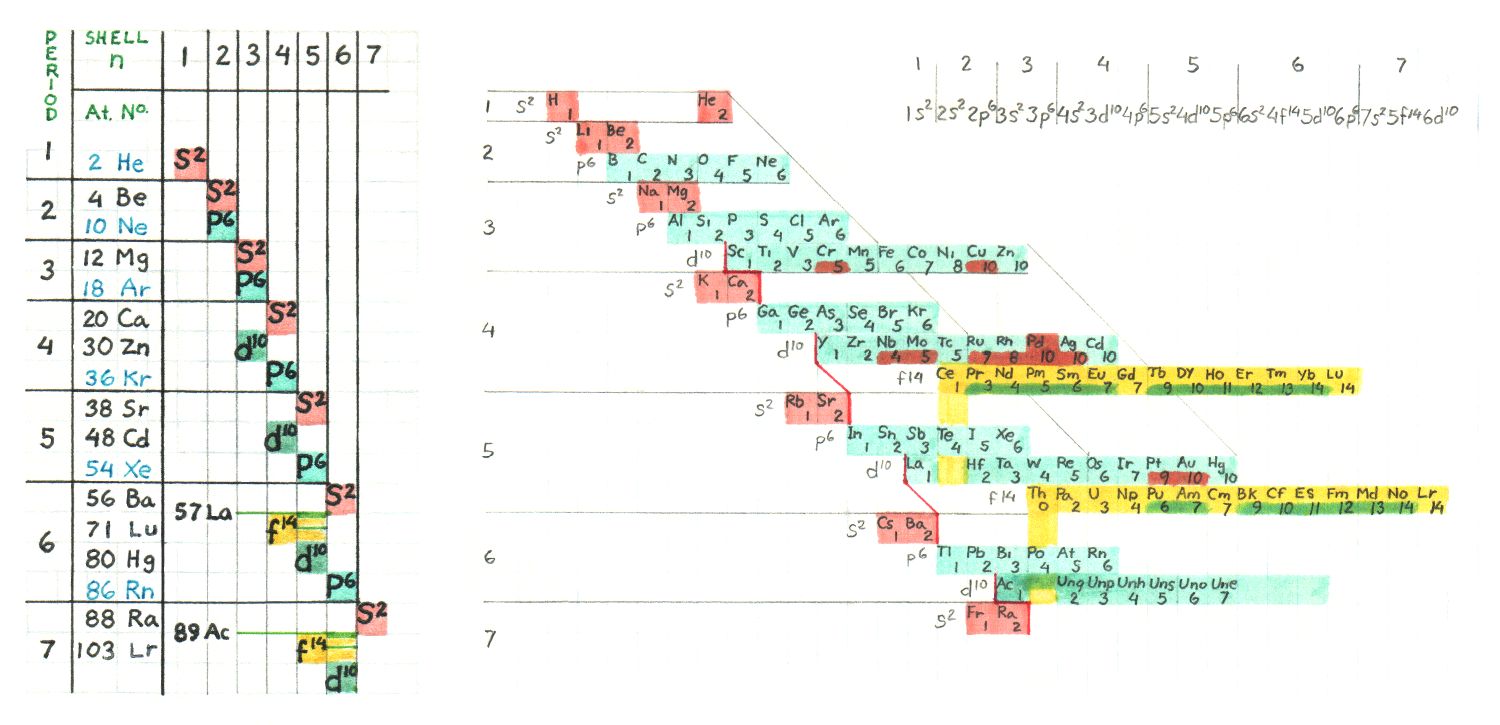
Electronic Orbital Periodicity
franklyn - 15-5-2008 at 02:53
Mendelevian grouping is only one possible organizational scheme, regardless of
the schematic choice. A table is useful only to the extent that it provides easy
reference to data and comparison. Most everyone who has considered arranging
elements in tabular form has pondered what layout best serves the purpose.
Below is a table I once made to determine the electronic shell and orbital structure
of any element at a glance. Everything to the left and above the elements position
indicates the complete full orbitals for those shells. Actually you can see the goup
memebers run diagonally from upper left to lower right
This arrangement shows that the progression of successive electrons is not straight
forward with regard to placement within the atoms. The Mendelevian sequence
begining with period 6 through the Lanthanides back to period 6 transition metals
until Radon, continuing with period 7 ending with the first member of the Actinides,
is as follows :
shell 6 , s orbital - Cesium, Barium ( Cs, Ba )
shell 5 , d orbital - Lanthanum ( La )
shell 4 , f orbital - Cerium -> Lutecium ( Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu )
shell 5 , d orbital - Hafnium -> Mercury ( Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg )
shell 6 , p orbital - Thallium -> Radon ( Tl, Pb, Bi, Po, At, Rn )
shell 7 , s orbital - Francium, Radium ( Fr, Ra )
shell 6 , d orbital - Actinium ( Ac )
shell 5 , f orbital - Thorium ( Th )
A scan of a periodic chart from 1979 crammed with element property data also
showing crystalline form, is in a zip file here _ http://www.badongo.com/file/9376819
Password = periodic
franklyn - 3-6-2010 at 17:19
http://www.sciencedaily.com/releases/2006/11/061119114755.ht...
.
franklyn - 30-4-2013 at 02:49
www.chemicool.com
Very detailed information of individual elements , select from table
The Internet Database of Periodic Tables
Oooh so many tables
www.meta-synthesis.com/webbook/35_pt/pt_database.php?Button=...
A sample _
Universal Periodic System of the Elements
www40.brinkster.com/maks47
Other papers
The Best Periodic Table of Elements
http://vixra.org/pdf/1302.0157v1.pdf
The following is the last part of the above paper
You must add - .pdf - on the end of the file to read it.
The Jiang Periodic Table Of The Elements
www.gsjournal.net/Science-Journals/Research Papers-Mathematical Physics/Download/4826
BAYEH's theoretical periodic table of elements
You must add - .pdf - on the end of the file to read it.
www.gsjournal.net/Science-Journals/Research%20Papers-Chemist...
Periodic Spiral
www.periodicspiral.com/periodic_spiral.pdf
www.periodicspiral.com
related thread
www.sciencemadness.org/talk/viewthread.php?tid=14494#pid1966...
.
kristofvagyok - 14-5-2013 at 10:38
Just found a "periodic spiral" on Tumblr:

Eddygp - 18-5-2013 at 05:30
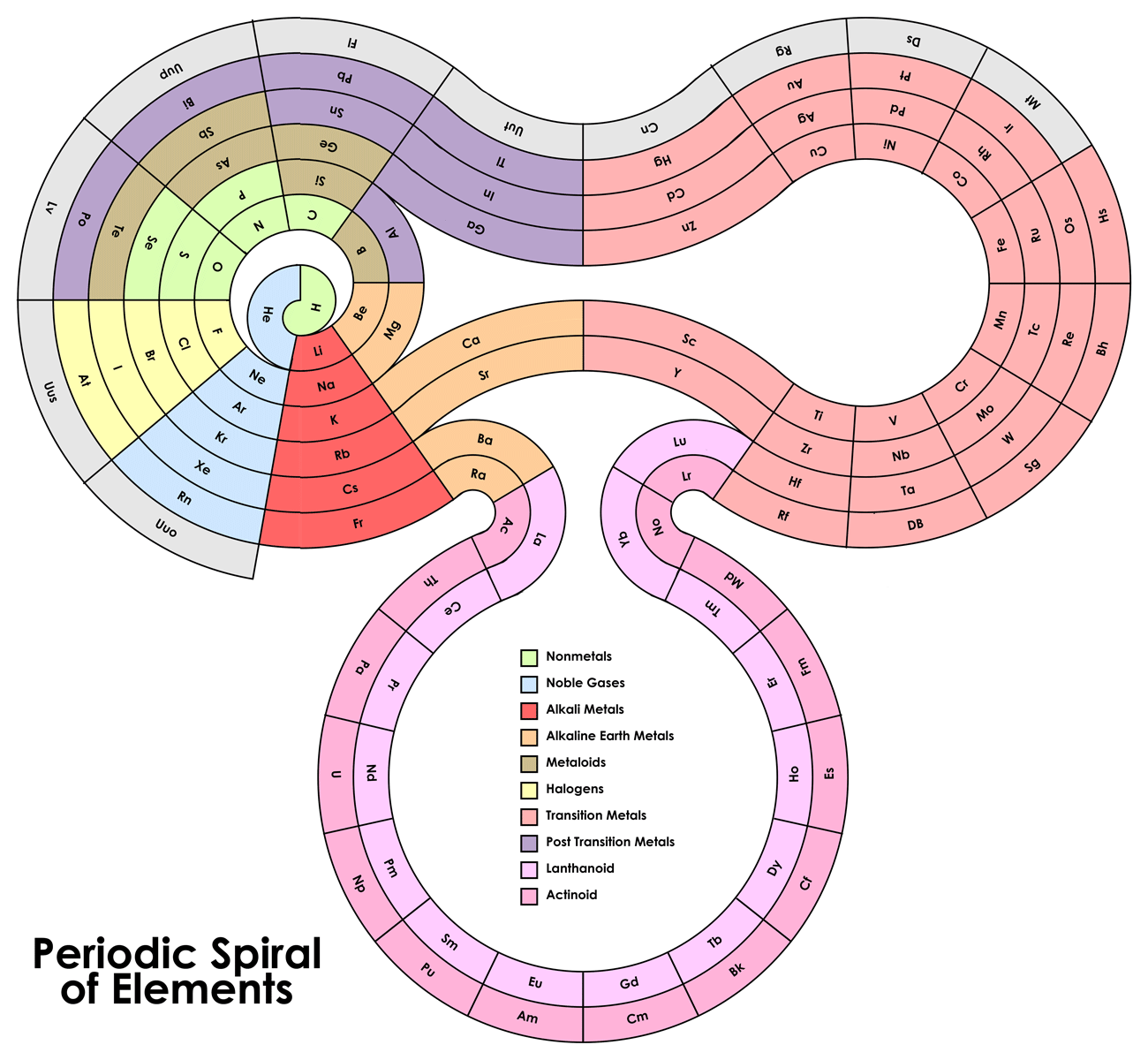
This one is fairly original.
However, after some thinking, I believe that the definitive periodic table/chart/whatever must be at least in 4 dimensions, because each time s, p, d,
f, etc. are added, the table is extended (that is why there is a vast space of transition metals and for lanthanides and actinides they usually pluck
them out of the table and put them below). In order to make this more visual (I mean, there is not such a leap between Be and B), 3-dimensions somehow
might do the job for a few orbitals, but there will be a point, with superactinides probably, that might require an extra dimension or really complex
twists.





 Then again, I could say the same about having connected Hydrogen to any column at all
Then again, I could say the same about having connected Hydrogen to any column at all 


