

Quote: Originally posted by White Yeti  |
 <sub>3</sub>Cl) on <a href="http://en.wikipedia.org/wiki/Baryte" target="_blank">Barite</a> <img
src="../scipics/_wiki.png" /> (BaSO<sub>4</sub>
<sub>3</sub>Cl) on <a href="http://en.wikipedia.org/wiki/Baryte" target="_blank">Barite</a> <img
src="../scipics/_wiki.png" /> (BaSO<sub>4</sub> </td></tr></table>
</td></tr></table>Quote: Originally posted by bfesser  |
Quote: Originally posted by bfesser  |
| Quote: |

Quote: Originally posted by bfesser  |
Quote: Originally posted by turd  |

 At least one sees a little bit of dichroism.
At least one sees a little bit of dichroism.
 Since I haven't used it for anything for a very long time, I'll grow
crystals and post pictures.
Since I haven't used it for anything for a very long time, I'll grow
crystals and post pictures.Quote: Originally posted by White Yeti  |

Quote: Originally posted by White Yeti  |
Quote: Originally posted by turd  |












Quote: Originally posted by White Yeti  |


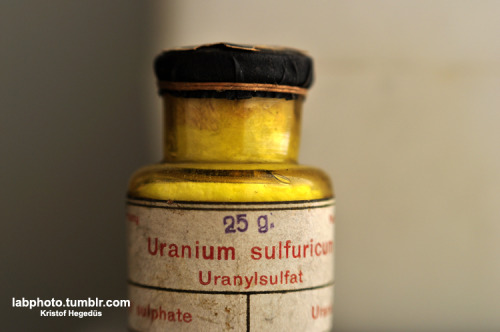

Quote: Originally posted by kristofvagyok  |




Quote: Originally posted by White Yeti  |

Quote: Originally posted by White Yeti  |

Quote: Originally posted by White Yeti  |
Quote: Originally posted by White Yeti  |
Quote: Originally posted by Endimion17  |
| Quote: |
 My newest solution to this problem
is to place a funnel over the opening of the jar. It's keeping the flies out, but the water doesn't seem to be evaporating too well. I might try to
use a fine mesh of some kind.
My newest solution to this problem
is to place a funnel over the opening of the jar. It's keeping the flies out, but the water doesn't seem to be evaporating too well. I might try to
use a fine mesh of some kind.







 Long needle crystals, looked quite nicer in person but you get the idea.
Long needle crystals, looked quite nicer in person but you get the idea. Quote: Originally posted by Wizzard  |






Quote: Originally posted by metalresearcher  |
Quote: Originally posted by adianadiadi  |
 LOL
LOLQuote: Originally posted by White Yeti  |

Quote: Originally posted by Endimion17  |
 they really do look awesome in bright light, so intricate and shiny, the
other batch came out more flakey and less "needle-ish". Not really photo worthy..
they really do look awesome in bright light, so intricate and shiny, the
other batch came out more flakey and less "needle-ish". Not really photo worthy..