

Quote: Originally posted by blogfast25  |
Quote: Originally posted by Sedit  |
 , with n = 4 for Cu and n = 6 for Ni … (2)
, with n = 4 for Cu and n = 6 for Ni … (2) and obtain values of 0.16
mol/l for the copper complex and 0.056 mol/l for the nickel complex. A significant difference, but, assuming these values are roughly correct, not a
great separation resolution…
and obtain values of 0.16
mol/l for the copper complex and 0.056 mol/l for the nickel complex. A significant difference, but, assuming these values are roughly correct, not a
great separation resolution…


Quote: Originally posted by Sedit  |
Quote: Originally posted by m1tanker78  |

Quote: Originally posted by Sedit  |
Quote: Originally posted by blogfast25  |
Quote: Originally posted by Sedit  |

Quote: Originally posted by blogfast25  |
 . Anyway, the hydroxide precipitate was green
. Anyway, the hydroxide precipitate was green I seen no strong evidence of Copper hydroxide which I find very encouraging meaning a final cleanse with Ammonia
hydroxide may be a moot point and unneeded.
I seen no strong evidence of Copper hydroxide which I find very encouraging meaning a final cleanse with Ammonia
hydroxide may be a moot point and unneeded. | Quote: |

 , My mind can now rest relatively peacefully
tonight. Now on to the next challenge of using this as nothing more then a final wash to remove trace Cu left over from the electro-deposition.
, My mind can now rest relatively peacefully
tonight. Now on to the next challenge of using this as nothing more then a final wash to remove trace Cu left over from the electro-deposition.
Quote: Originally posted by Sedit  |


 . Beer taste so much worse in my mouth the second day.
. Beer taste so much worse in my mouth the second day.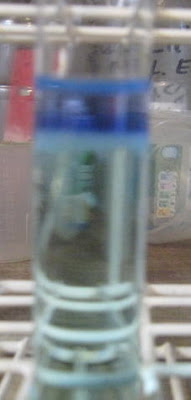
 I do honestly appreciate your efforts
without a doubt. Many times people will discredit something and never once head to the lab to see for themselves and the fact you did quickly earned
my respect without a doubt. You seen a claim you did not feel was valid and at the speed of lightning setup to reproduce the experiment. That's not
just a good scientist that's someone with a love and a passion for what they do ::End Kissing ass::
I do honestly appreciate your efforts
without a doubt. Many times people will discredit something and never once head to the lab to see for themselves and the fact you did quickly earned
my respect without a doubt. You seen a claim you did not feel was valid and at the speed of lightning setup to reproduce the experiment. That's not
just a good scientist that's someone with a love and a passion for what they do ::End Kissing ass::
Quote: Originally posted by cyanureeves  |

Quote: Originally posted by m1tanker78  |
Quote: Originally posted by blogfast25  |


Quote: Originally posted by Sedit  |
 - ½ log [Ni2+])
- ½ log [Ni2+])

Quote: Originally posted by blogfast25  |
| Quote: |
 then form and these are emerald green. But it needs quite a lot of
Cl<sup>-</sup>. My solutions (above) are in fact mixed CuCl2/NiCl2 and they're blue. The complexation constant of the chlorocuprate
complex is fairly small.
then form and these are emerald green. But it needs quite a lot of
Cl<sup>-</sup>. My solutions (above) are in fact mixed CuCl2/NiCl2 and they're blue. The complexation constant of the chlorocuprate
complex is fairly small.
| Quote: |



Quote: Originally posted by blogfast25  |
| Quote: |
Quote: Originally posted by m1tanker78  |

 As if this whole set of nonsense couldn't get much stranger to the
chemist this happens.
As if this whole set of nonsense couldn't get much stranger to the
chemist this happens.



Quote: Originally posted by Sedit  |




Quote: Originally posted by blogfast25  |
Quote: Originally posted by Sedit  |
 I've read a few accounts of people using AscA to
precipitate colloidal copper from a Cu2+ solution but no mention of other metals (nickel in this case). What am I doing wrong?
I've read a few accounts of people using AscA to
precipitate colloidal copper from a Cu2+ solution but no mention of other metals (nickel in this case). What am I doing wrong? ) I will post as many pictures as I can.
) I will post as many pictures as I can.Quote: Originally posted by Formatik  |


Quote: Originally posted by m1tanker78  |
Quote: Originally posted by Formatik  |
Quote: Originally posted by m1tanker78  |
Quote: Originally posted by m1tanker78  |
Quote: Originally posted by m1tanker78  |
Quote: Originally posted by cyanureeves  |
| Quote: |
| Quote: |
| Quote: |

Quote: Originally posted by m1tanker78  |
Quote: Originally posted by m1tanker78  |
Quote: Originally posted by m1tanker78  |
Quote: Originally posted by cyanureeves  |
Quote: Originally posted by blogfast25  |
Quote: Originally posted by Hexavalent  |
Quote: Originally posted by cyanureeves  |
Quote: Originally posted by blogfast25  |
Quote: Originally posted by cyanureeves  |
Quote: Originally posted by Mixell  |
Quote: Originally posted by tetrahedron  |