Eucalyptol
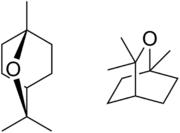
| |
| Names | |
|---|---|
| IUPAC name
1,3,3-Trimethyl-2-oxabicyclo[2.2.2]octane
| |
| Other names
1,8-Cineole
1,8-Epoxy-p-menthane | |
| Properties | |
| C10H18O | |
| Molar mass | 154.249 g/mol |
| Appearance | Colorless liquid |
| Odor | Mint or camphor-like |
| Density | 0.9225 g/cm3 |
| Melting point | 2.9 °C (37.2 °F; 276.0 K) |
| Boiling point | 176–177 °C (349–351 °F; 449–450 K) |
| 0.35 g/100 ml (20 °C) | |
| Solubility | Miscible with glacial acetic acid, chloroform, diethyl ether, ethanol, glycerol, propylene glycol Slightly soluble in CCl4 |
| Vapor pressure | 1.90 mm Hg at 25 °C |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 49 °C (120 °F; 322 K) |
| Related compounds | |
| Related compounds
|
Menthol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Eucalyptol is a natural organic compound that is a colorless liquid. It is a cyclic ether and a monoterpenoid.
Contents
Properties
Chemical
Eucalyptol forms crystalline adducts with hydrohalic acids, o-cresol, resorcinol and phosphoric acid. Formation of these adducts is useful for purification.
Physical
Eucalyptol is a clear viscous liquid, with a fresh mint-like smell and a spicy, cooling taste. It is insoluble in water, but miscible with chloroform, diethyl ether and ethanol. The boiling point of this compound is 176 °C and the flash point is 49 °C.
Availability
Eucalyptol comprises up to 90% of eucalyptus oil's mass. Though not very cheap, it is a readily available source.
Purification can be achieved via fractional or vacuum distillation.
Preparation
It's much cheaper to extract the product from eucalyptus oil than synthesizing it from scratch.
Projects
- Alcohol catalyzed alkali metal production
- Insecticide and insect repellent
- Flavouring agent
Handling
Safety
Eucalyptol has low toxicity in small amounts. In higher-than-normal doses, eucalyptol is hazardous via ingestion, skin contact, or inhalation. It can have acute health effects on behavior, the respiratory tract, and the nervous system.
Storage
In closed bottles, away from light and air.
Disposal
No special disposal is required, as it's a naturally-occuring compound that has low toxicity.
Can be burned if desired.