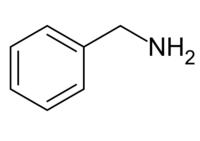
Benzylamine
| |
| Names | |
|---|---|
| IUPAC name
1-Phenylmethanamine
| |
| Other names
Benzyl amine
Phenylmethylamine α-Aminotoluene | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C7H9N C6H5CH2NH2 | |
| Molar mass | 107.156 g/mol |
| Appearance | Colorless to slight yellowish liquid |
| Odor | Amine-like |
| Density | 0.981 g/cm3 (20 °C) |
| Melting point | 10 °C (50 °F; 283 K) |
| Boiling point | 185 °C (365 °F; 458 K) |
| Miscible | |
| Solubility | Miscible in acetone, benzene, diethyl ether, ethanol, methanol, pyridine Partially miscible with chloroform |
| Vapor pressure | 0.662 mmHg at 25 °C |
| Acidity (pKa) | 9.34 |
| Thermochemistry | |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 65 °C (149 °F; 338 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
552 mg/kg (rat, oral) |
| Related compounds | |
| Related compounds
|
Aniline |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benzylamine is an organic chemical compound with the chemical formula C6H5CH2NH2 or C7H9N (sometimes abbreviated as PhCH2NH2 or BnNH2).
Contents
Properties
Chemical
Benzylamine reacts with acids forming salts.
Benzylamine reacts with acetyl chloride to form N-benzylacetamide, an exemplar of the Schotten–Baumann reaction.
Physical
Benzylamine is a colorless viscous liquid that may turn slightly yellowish after prolonged contact with air. It is miscible with water and many organic solvents.
Availability
Benzylamine is sold by chemical suppliers in both liquid form and as salt.
Preparation
There are several routes for benzylamine.
The main industrial route being the reaction of benzyl chloride and ammonia. The reaction alone produces sufficient heat to maintain the temperature between 30-34 °C.[1]
Benzylamine can also be produced in the lab by the reduction of benzonitrile with Raney nickel, and reductive amination of benzaldehyde with formamide or ammonium formate (Leuckart reaction).
Projects
- Make anti-nausea medication
- Make Hexanitrohexaazaisowurtzitane (HNIW)
Handling
Safety
Benzylamine, while irritant, exhibits modest oral toxicity in rats, with LD50 of 552-1,130 mg/kg. It is readily biodegraded.[2]
Storage
Benzylamine should be kept in airtight bottles, away from air and light, if kept in liquid form. The salt form is somewhat more stable to oxidation, though not by much.
Disposal
Benzylamine should be mixed with a more flammable solvent and burned in an incinerator or outside.
References
- ↑ https://erowid.org/archive/rhodium/chemistry/benzylamine.html
- ↑ https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a04_009.pub2
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Aromatic compounds
- Amines
- Primary amines
- Bases
- Organic bases
- Lewis bases
- Materials unstable in acidic solution
- Foul smelling compounds
- Liquids
- Irritants
- Air-sensitive materials