Difference between revisions of "Acetaldehyde"
(Created page with "152x152px '''Acetaldehyde''' is the organic compound with chemical formula CH<sub>3</sub>CHO. It is the second-simplest aldehyde and find...") |
|||
| Line 4: | Line 4: | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | |||
Depending on reaction conditions, the oxidation of acetaldehyde by oxygen variously co-produces [[acetic anhydride]] and [[acetic acid]] via the intermediate [[peracetic acid]]. The process relies on a catalyst containing metal ions and is typically conducted by introduction of gaseous oxygen into liquid acetaldehyde. | Depending on reaction conditions, the oxidation of acetaldehyde by oxygen variously co-produces [[acetic anhydride]] and [[acetic acid]] via the intermediate [[peracetic acid]]. The process relies on a catalyst containing metal ions and is typically conducted by introduction of gaseous oxygen into liquid acetaldehyde. | ||
| Line 16: | Line 15: | ||
Industrially, acetaldehyde is produced via the oxidation of [[ethylene|ethene]] over a copper-palladium catalyst. Two routes exist from ethanol, the first an exothermic, self-sustaining oxidation reaction using a copper or silver catalyst at a temperature of 500-650°C: | Industrially, acetaldehyde is produced via the oxidation of [[ethylene|ethene]] over a copper-palladium catalyst. Two routes exist from ethanol, the first an exothermic, self-sustaining oxidation reaction using a copper or silver catalyst at a temperature of 500-650°C: | ||
| − | + | :2 CH<sub>3</sub>CH<sub>2</sub>OH + O<sub>2</sub> → 2 CH<sub>3</sub>CHO + 2 H<sub>2</sub>O | |
The second method of preparing acetaldehyde from ethanol involves its dehydrogenation at a temperature of of 260-290 °C, again over a catalyst of copper. This route is endothermic, requiring constant and uniform heating, but has the advantage of not requiring oxygen input, which also reduces the risk of fire. | The second method of preparing acetaldehyde from ethanol involves its dehydrogenation at a temperature of of 260-290 °C, again over a catalyst of copper. This route is endothermic, requiring constant and uniform heating, but has the advantage of not requiring oxygen input, which also reduces the risk of fire. | ||
| − | CH<sub>3</sub>CH<sub>2</sub>OH → CH<sub>3</sub>CHO + H<sub>2</sub><ref>Ullmann's Encyclopedia Of Industrial Chemistry, Wiley-VCH (2007)</ref> | + | :CH<sub>3</sub>CH<sub>2</sub>OH → CH<sub>3</sub>CHO + H<sub>2</sub><ref>Ullmann's Encyclopedia Of Industrial Chemistry, Wiley-VCH (2007)</ref> |
==Projects== | ==Projects== | ||
| Line 38: | Line 37: | ||
<references/> | <references/> | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
| + | |||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
[[Category:Organic compounds]] | [[Category:Organic compounds]] | ||
[[Category:Aldehydes]] | [[Category:Aldehydes]] | ||
| + | [[Category:Volatile chemicals]] | ||
[[Category:Fragrant compounds]] | [[Category:Fragrant compounds]] | ||
| + | [[Category:Carcinogenic]] | ||
Revision as of 15:57, 20 September 2015
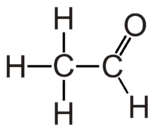
Acetaldehyde is the organic compound with chemical formula CH3CHO. It is the second-simplest aldehyde and finds use as a building block in organic synthesis.
Contents
Properties
Chemical
Depending on reaction conditions, the oxidation of acetaldehyde by oxygen variously co-produces acetic anhydride and acetic acid via the intermediate peracetic acid. The process relies on a catalyst containing metal ions and is typically conducted by introduction of gaseous oxygen into liquid acetaldehyde.
Physical
Acetaldehyde is a transparent, volatile, and extremely flammable liquid at room temperature that boils at only 20.2°C (68.4°F), making it difficult to store. The characteristic odor of acetaldehyde is sweet and reminiscent of green apple. Acetaldehyde has a flash point of only −39°C, making it absolutely crucial that any significant amount is kept away from possible ignition sources.
Availability
No over-the-counter source of acetaldehyde is known, and sources are unlikely due to the difficulty and danger of prolonged storage.
Preparation
Industrially, acetaldehyde is produced via the oxidation of ethene over a copper-palladium catalyst. Two routes exist from ethanol, the first an exothermic, self-sustaining oxidation reaction using a copper or silver catalyst at a temperature of 500-650°C:
- 2 CH3CH2OH + O2 → 2 CH3CHO + 2 H2O
The second method of preparing acetaldehyde from ethanol involves its dehydrogenation at a temperature of of 260-290 °C, again over a catalyst of copper. This route is endothermic, requiring constant and uniform heating, but has the advantage of not requiring oxygen input, which also reduces the risk of fire.
- CH3CH2OH → CH3CHO + H2[1]
Projects
- Pentaerythritol synthesis
Handling
Safety
Acetaldehyde is designated a probable carcinogen and is many times more toxic than ethanol, which it is a metabolite of. With a high chance of vaporization and a flash point of only −39 °C, acetaldehyde represents a significant fire hazard, and care should be made that all potential sources of ignition are kept away.
Storage
Acetaldehyde must be stored in a highly temperature-controlled environment given its low boiling point, and should also be kept in a separate cabinet or secondary container in a well-ventilated space, always away from potential sources of ignition.
Disposal
Acetaldehyde can be safely burned.
References
- ↑ Ullmann's Encyclopedia Of Industrial Chemistry, Wiley-VCH (2007)