Difference between revisions of "Phenanthroline"
| Line 5: | Line 5: | ||
| PIN = | | PIN = | ||
| SystematicName = | | SystematicName = | ||
| − | | OtherNames = 4,5-Diazaphenanthrene<br>O-phenanthroline | + | | OtherNames = 4,5-Diazaphenanthrene<br>O-phenanthroline<br>Phen |
<!-- Images --> | <!-- Images --> | ||
| ImageFile = Phenanthroline bottle sample.jpg | | ImageFile = Phenanthroline bottle sample.jpg | ||
| Line 62: | Line 62: | ||
| MeltingPt_ref = | | MeltingPt_ref = | ||
| MeltingPt_notes = | | MeltingPt_notes = | ||
| + | | Odor = Odorless | ||
| pKa = 4.86 (phenH<sup>+</sup>) | | pKa = 4.86 (phenH<sup>+</sup>) | ||
| pKb = | | pKb = | ||
| Line 67: | Line 68: | ||
| SolubleOther = Soluble in [[acetone]], [[ethanol]] | | SolubleOther = Soluble in [[acetone]], [[ethanol]] | ||
| Solvent = | | Solvent = | ||
| − | | VaporPressure = | + | | VaporPressure = ~0 mmHg |
}} | }} | ||
| Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| Line 117: | Line 118: | ||
===Physical=== | ===Physical=== | ||
| − | Phenanthroline is a white solid, poorly soluble in water, but more soluble in organic solvents, such as primary alcohols and acetone. | + | Phenanthroline is a white solid, odorless, poorly soluble in water, but more soluble in organic solvents, such as primary alcohols and acetone. |
==Availability== | ==Availability== | ||
| − | Can be purchased online. | + | Can be purchased online, though it's expensive. |
==Preparation== | ==Preparation== | ||
| Line 127: | Line 128: | ||
==Projects== | ==Projects== | ||
*Make [[ferroin]] | *Make [[ferroin]] | ||
| − | *Belousov–Zhabotinsky reaction | + | *[[Belousov–Zhabotinsky reaction]] |
*Alkyllithium reagent indicator | *Alkyllithium reagent indicator | ||
| Line 138: | Line 139: | ||
===Disposal=== | ===Disposal=== | ||
| − | Can be destroyed with a strong oxidizer, like [[Fenton's reagent]] or [[piranha solution]] by slowly adding it in the oxidizing mixture, followed by neutralization and then poured down the drain. | + | Can be safely destroyed with a strong oxidizer, like [[Fenton's reagent]] or [[piranha solution]]. This can be done by slowly adding it in the oxidizing mixture, followed by neutralization and then poured down the drain. |
==References== | ==References== | ||
| Line 144: | Line 145: | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
*[http://www.sciencemadness.org/talk/viewthread.php?tid=12537 Dicyanobis(1,10-phenanthrolin)Fe(II)] | *[http://www.sciencemadness.org/talk/viewthread.php?tid=12537 Dicyanobis(1,10-phenanthrolin)Fe(II)] | ||
| + | *[https://www.sciencemadness.org/whisper/viewthread.php?tid=158879 Molybdenum III - Phenanthroline complex (?)] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
Latest revision as of 20:22, 16 October 2022
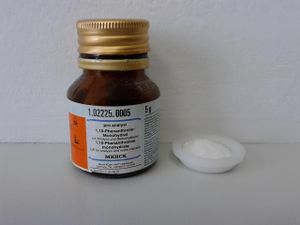 Phenanthroline monohydrate sample
| |
| Names | |
|---|---|
| IUPAC name
1,10-phenanthroline
| |
| Other names
4,5-Diazaphenanthrene
O-phenanthroline Phen | |
| Properties | |
| C12H8N2 | |
| Molar mass | 180.21 g/mol |
| Appearance | Colorless crystalline solid |
| Odor | Odorless |
| Density | 1.31 g/cm3 |
| Melting point | 117 °C (243 °F; 390 K) |
| Boiling point | 300 °C (572 °F; 573 K) |
| 0.269 g/100 ml (at 25 °C) | |
| Solubility | Soluble in acetone, ethanol |
| Vapor pressure | ~0 mmHg |
| Acidity (pKa) | 4.86 (phenH+) |
| Hazards | |
| Safety data sheet | AcrosOrganics |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phenanthroline or 1,10-phenanthroline is a heterocyclic organic compound, used as a ligand in coordination chemistry, as it forms strong complexes with many metal ions.
Contents
Properties
Chemical
Addition of phenanthroline to iron(III) sulfate in water gives ferroin.
- 3 1,10-Phen + Fe2+ → [Fe(1,10-Phen)3]2+
Physical
Phenanthroline is a white solid, odorless, poorly soluble in water, but more soluble in organic solvents, such as primary alcohols and acetone.
Availability
Can be purchased online, though it's expensive.
Preparation
Can be made by two successive Skraup reactions of glycerol with o-phenylenediamine, catalyzed by conc. sulfuric acid, and a selective oxidizing agent, such as aqueous arsenic acid or nitrobenzene. Dehydration of glycerol gives acrolein, which condenses with the amine followed by a cyclization.
Projects
- Make ferroin
- Belousov–Zhabotinsky reaction
- Alkyllithium reagent indicator
Handling
Safety
Phenanthroline is irritant and harmful. It is considered a mild neurotoxin, strong nephrotoxin, and powerful diuretic.
Storage
In closed bottles, away from corrosive vapors.
Disposal
Can be safely destroyed with a strong oxidizer, like Fenton's reagent or piranha solution. This can be done by slowly adding it in the oxidizing mixture, followed by neutralization and then poured down the drain.