Difference between revisions of "Trimethyl borate"
(→Preparation) |
Diachrynic (Talk | contribs) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 139: | Line 139: | ||
===Disposal=== | ===Disposal=== | ||
Trimethyl borate can be safely burned. It will hydrolyze in water to yield methanol and boric acid, which can be safely disposed of. | Trimethyl borate can be safely burned. It will hydrolyze in water to yield methanol and boric acid, which can be safely disposed of. | ||
| + | |||
| + | ==Gallery== | ||
| + | <gallery widths="200" position="center" columns="4" orientation="none"> | ||
| + | Trimethyl_borate_methanol_azeotrope.jpg|Trimethyl borate as the methanol azeotrope | ||
| + | Trimethyl_borate_flame.jpg|Flame color of trimethyl borate | ||
| + | </gallery> | ||
==See also== | ==See also== | ||
| Line 159: | Line 165: | ||
[[Category:Materials that react with water]] | [[Category:Materials that react with water]] | ||
[[Category:Easily prepared chemicals]] | [[Category:Easily prepared chemicals]] | ||
| + | [[Category:Liquids]] | ||
Latest revision as of 19:50, 9 September 2021
 Azeotrope of 75% trimethyl borate and 25% methanol
| |
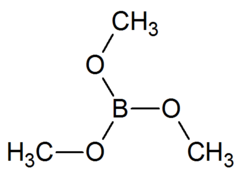 Structure of the compound
| |
| Names | |
|---|---|
| IUPAC name
Trimethyl borate
| |
| Other names
Boron trimethoxide
Trimethoxyborane Trimethoxyboron | |
| Properties | |
| C3H9BO3 | |
| Molar mass | 103.91 g/mol |
| Appearance | Colorless liquid |
| Density | 0.932 g/cm3 |
| Melting point | −34 °C (−29 °F; 239 K) |
| Boiling point | 68 °C (154 °F; 341 K) |
| Reacts | |
| Solubility | Reacts with carboxylic acids Miscible with acetone, diethyl ether, ethanol, heptane, hexane, isopropanol, isopropylamine, methanol, mineral oil, THF |
| Vapor pressure | 137 mmHg at 25 °C |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | -8 °C |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
6.140 mg/kg (rat, oral) 1.845 mg/kg (rabbit, dermal) |
| Related compounds | |
| Related compounds
|
Triethyl borate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trimethyl borate is a boron triester consisting of a single boron atom bound to three methoxide groups. It is a volatile liquid that ignites easily to give a brilliant green flame, purer than that of barium. It may be produced readily in large quantities.
Contents
Properties
Chemical
Trimethyl borate, being produced by dehydration, decomposes into methanol and boric acid on exposure to water. It burns in the presence of oxygen to form boron trioxide. In flames, it emits a brilliant green color that overpowers many other flame colors, as evidenced here.
Physical
Trimethyl borate is a colorless liquid at room temperature, that evaporates easily. It has a melting point of −34 °C and boils at 68 °C. It will decompose in water and acids.
Availability
Relatively pure trimethyl borate can be bought from only a few chemical suppliers as it has few commercial applications. It is by far cheaper and more practical to produce it.
Preparation
Trimethyl borate can be produced by adding an excess of dry methanol to boric acid or, preferably, boric oxide, with a small amount of sulfuric acid and/or some heating to dehydrate the mixture if needed. Since an excess of methanol is used, the final product will be an azeotropic mixture of methanol (25%) and trimethyl borate (75%). It's possible to obtain pure trimethyl borate by turning the methanol into trimethyl borate using a boron trihalide, such as boron tribromide. However, the trihalide should be added slowly to prevent the already present boron tribromide from hydrolyzing.
Projects
- Green flames
- Making sodium borohydride
Handling
Safety
There is little data on the toxicity of trimethyl borate. Nevertheless, it is a good idea not to consume or inhale it, and its hydrolysis products aren't exactly healthy. Keep away from open flame. Water may be used to put out trimethyl borate flames. When igniting trimethyl borate, goggles should be worn to prevent boric oxide dust from irritating the eyes.
Storage
Trimethyl borate should only be stored in closed bottles, away from light or any heat source. Since it is sensitive to water, it's best to keep the bottle airtight or put it into a desiccator.
Disposal
Trimethyl borate can be safely burned. It will hydrolyze in water to yield methanol and boric acid, which can be safely disposed of.

