Difference between revisions of "Ethyl acetate"
(→Relevant Sciencemadness threads) |
|||
| (12 intermediate revisions by the same user not shown) | |||
| Line 5: | Line 5: | ||
| PIN = | | PIN = | ||
| SystematicName = Ethyl acetate | | SystematicName = Ethyl acetate | ||
| − | | OtherNames = | + | | OtherNames = |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = Ethyl acetate structure.png |
| − | | ImageSize = | + | | ImageSize = 250 |
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| Line 58: | Line 53: | ||
| BoilingPt_notes = | | BoilingPt_notes = | ||
| Density = 0.902 g/ml | | Density = 0.902 g/ml | ||
| − | | Formula = CH<sub>3</sub>COOCH<sub>2</sub>CH<sub>3</sub> | + | | Formula = C<sub>4</sub>H<sub>8</sub>O<sub>2</sub><br>CH<sub>3</sub>COOCH<sub>2</sub>CH<sub>3</sub> |
| HenryConstant = | | HenryConstant = | ||
| LogP = | | LogP = | ||
| − | | MolarMass = 88. | + | | MolarMass = 88.106 g/mol |
| MeltingPt = | | MeltingPt = | ||
| MeltingPtC = -83.6 | | MeltingPtC = -83.6 | ||
| MeltingPt_ref = | | MeltingPt_ref = | ||
| MeltingPt_notes = | | MeltingPt_notes = | ||
| − | | Odor = Fruity, | + | | Odor = Fruity, pear-like |
| pKa = 25 | | pKa = 25 | ||
| pKb = | | pKb = | ||
| Solubility = 8.3g/100ml | | Solubility = 8.3g/100ml | ||
| − | | SolubleOther = | + | | SolubleOther = Miscible with [[acetone]], [[alcohol]]s, [[benzene]], [[chloroform]], [[diethyl ether]], [[ethanol]], [[toluene]], [[xylene]] |
| Solvent = | | Solvent = | ||
| − | | VaporPressure = | + | | VaporPressure = 92.3 mmHg (20 °C) |
}} | }} | ||
| Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| Line 93: | Line 88: | ||
}} | }} | ||
| Section6 = {{Chembox Hazards | | Section6 = {{Chembox Hazards | ||
| − | | AutoignitionPt = | + | | AutoignitionPt = 427 °C (800.6 °F; 700 K) |
| − | | ExploLimits = | + | | ExploLimits = 2.0–11.5% |
| − | | ExternalMSDS = | + | | ExternalMSDS = [https://www.docdroid.net/945M2rK/ethyl-acetate-sa.pdf.html Sigma-Aldrich] |
| − | | FlashPt = | + | | FlashPt = −4 °C (25 °F; 269 K) |
| − | | LD50 = | + | | LD50 = 11,300 mg/kg (rat, oral) |
| − | | LC50 = | + | | LC50 = 16,000 ppm (rat, 6 h)<br>12,295 ppm (mouse, 2 h)<br>1600 ppm (rat, 8 h) |
| − | | MainHazards = | + | | MainHazards = Flammable |
| NFPA-F = | | NFPA-F = | ||
| NFPA-H = | | NFPA-H = | ||
| Line 113: | Line 108: | ||
}} | }} | ||
}} | }} | ||
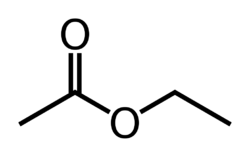
| − | + | '''Ethyl acetate''' or '''ethyl ethanoate''' is the organic compound with the chemical formula '''CH<sub>3</sub>-COO-CH<sub>2</sub>-CH<sub>3</sub>'''. The acetate [[ester]] of [[ethanol]], ethyl acetate is a safe and common laboratory and domestic solvent with a distinctive odor. | |
| − | '''Ethyl acetate''' or '''ethyl ethanoate''' is the organic compound with the chemical formula CH<sub>3</sub>-COO-CH<sub>2</sub>-CH<sub>3</sub>. The acetate [[ester]] of [[ethanol]], ethyl acetate is a safe and common laboratory and domestic solvent with a distinctive odor. | + | |
==Properties== | ==Properties== | ||
| Line 124: | Line 118: | ||
==Availability== | ==Availability== | ||
| − | Ethyl acetate can often be found in hardware stores either in pure form as a solvent(sometimes labelled as [[Methyl ethyl ketone|MEK]] substitute) or as a component of mixed solvents, which may or may not allow its extraction via fractional distillation. Many acetone-free nail polish removers often contain it as well. Some types of paint thinners contain a mixture of ethyl acetate and methyl acetate. | + | Ethyl acetate can often be found in hardware stores either in pure form as a solvent (sometimes labelled as [[Methyl ethyl ketone|MEK]] substitute) or as a component of mixed solvents, which may or may not allow its extraction via fractional distillation. Many acetone-free nail polish removers often contain it as well, usually mixed with alcohol, water, glycerol and other long chain esters. Some types of paint thinners and polyurethane solvents contain a mixture of ethyl acetate and [[methyl acetate]], usually in a 1:1 ratio. Both solvents can be extracted by using fractional distillation. |
| + | |||
| + | In some countries, the sale of pure or lab-grade ethyl acetate may be monitored, due to its use in the production of phenylacetone or purification of various drugs, though this route has not been used in decades by meth cooks, who have largely switched to the pseudoephedrine reduction route. | ||
==Preparation== | ==Preparation== | ||
| Line 138: | Line 134: | ||
===Storage=== | ===Storage=== | ||
| − | Ethyl acetate can be stored relatively indefinitely, but may degrade over time in the presence of substantial water. | + | Ethyl acetate can be stored relatively indefinitely, but may degrade over time in the presence of substantial water or bases. |
===Disposal=== | ===Disposal=== | ||
| Line 146: | Line 142: | ||
<references/> | <references/> | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=13298 Ethyl Acetate?] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
[[Category:Organic compounds]] | [[Category:Organic compounds]] | ||
[[Category:Esters]] | [[Category:Esters]] | ||
| − | |||
[[Category:Solvents]] | [[Category:Solvents]] | ||
| + | [[Category:Polar solvents]] | ||
[[Category:Aprotic solvents]] | [[Category:Aprotic solvents]] | ||
[[Category:Fragrant compounds]] | [[Category:Fragrant compounds]] | ||
[[Category:Materials unstable in basic solution]] | [[Category:Materials unstable in basic solution]] | ||
[[Category:Liquids]] | [[Category:Liquids]] | ||
| + | [[Category:Readily available chemicals]] | ||
| + | [[Category:Essential reagents]] | ||
Latest revision as of 18:00, 5 August 2019
| |
| Names | |
|---|---|
| IUPAC name
Ethyl acetate
| |
| Systematic IUPAC name
Ethyl acetate | |
| Identifiers | |
| Properties | |
| C4H8O2 CH3COOCH2CH3 | |
| Molar mass | 88.106 g/mol |
| Appearance | Colorless liquid |
| Odor | Fruity, pear-like |
| Density | 0.902 g/ml |
| Melting point | −83.6 °C (−118.5 °F; 189.6 K) |
| Boiling point | 78.1 °C (172.6 °F; 351.2 K) |
| 8.3g/100ml | |
| Solubility | Miscible with acetone, alcohols, benzene, chloroform, diethyl ether, ethanol, toluene, xylene |
| Vapor pressure | 92.3 mmHg (20 °C) |
| Acidity (pKa) | 25 |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | −4 °C (25 °F; 269 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
11,300 mg/kg (rat, oral) |
| LC50 (Median concentration)
|
16,000 ppm (rat, 6 h) 12,295 ppm (mouse, 2 h) 1600 ppm (rat, 8 h) |
| Related compounds | |
| Related compounds
|
Ethyl formate Methyl acetate Propyl acetate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethyl acetate or ethyl ethanoate is the organic compound with the chemical formula CH3-COO-CH2-CH3. The acetate ester of ethanol, ethyl acetate is a safe and common laboratory and domestic solvent with a distinctive odor.
Contents
Properties
Chemical
Ethyl acetate, like many other carboxylate esters, is prone to hydrolysis accelerated by basic conditions. Because ethyl acetate is cheap and easy to come by in some locations, it is sometimes intentionally hydrolyzed using sodium hydroxide into its component species: acetic acid and ethanol. The acetic acid produced reacts with the sodium hydroxide to form sodium acetate, effectively providing two useful and easily separable reagents. Because of it's polar yet aprotic nature, ethyl acetate is a commonly used solvent in chromatography.
Physical
Ethyl acetate is a clear, colorless, mobile liquid at room temperature. As with other carboxylate esters, ethyl acetate has an ethereal, fruity smell, reminiscent to that of pears. Its chief use as a solvent comes from its ability to dissolve many water-insoluble substances, such as glues and lacquers, and it is often used as a substitute for another popular solvent in hardware stores, methyl ethyl ketone.
Availability
Ethyl acetate can often be found in hardware stores either in pure form as a solvent (sometimes labelled as MEK substitute) or as a component of mixed solvents, which may or may not allow its extraction via fractional distillation. Many acetone-free nail polish removers often contain it as well, usually mixed with alcohol, water, glycerol and other long chain esters. Some types of paint thinners and polyurethane solvents contain a mixture of ethyl acetate and methyl acetate, usually in a 1:1 ratio. Both solvents can be extracted by using fractional distillation.
In some countries, the sale of pure or lab-grade ethyl acetate may be monitored, due to its use in the production of phenylacetone or purification of various drugs, though this route has not been used in decades by meth cooks, who have largely switched to the pseudoephedrine reduction route.
Preparation
Ethyl acetate is most often prepared by the Fischer esterification between ethanol and glacial acetic acid. Using anhydrous reagents and sulfuric acid as a catalyst and drying agent results in a roughly 65% yield. As with most such syntheses, this esterification is conducted under reflux and is benefited by the removal of water by some means, such as excess sulfuric acid or a desiccant. The ethyl acetate formed is difficult to remove from any remaining ethanol, though one way to do so is by dumping the products of the reaction into water, in which ethyl acetate is not highly soluble, forming a layer on top which can be separated off physically. The crude ethyl acetate is then washed with sodium bicarbonate solution to remove residual acids and gently distilled over a desiccant such as calcium chloride.
Projects
- Caffeine extraction
- Ethyl acetoacetate synthesis
Handling
Safety
Ethyl acetate is flammable as well as being a mild skin irritant, but is relatively nontoxic to humans.
Storage
Ethyl acetate can be stored relatively indefinitely, but may degrade over time in the presence of substantial water or bases.
Disposal
Ethyl acetate does not have any special requirements for disposal, but it may be a better idea to recover it via distillation or hydrolyze it to useful ethanol and acetic acid instead.
References
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Esters
- Solvents
- Polar solvents
- Aprotic solvents
- Fragrant compounds
- Materials unstable in basic solution
- Liquids
- Readily available chemicals
- Essential reagents