Difference between revisions of "Methane"
| (4 intermediate revisions by 2 users not shown) | |||
| Line 8: | Line 8: | ||
| OtherNames = Biogas<br>Carbon tetrahydride<br>Hydrogen carbide<br>Marsh gas<br>Methyl hydride<br>Natural gas | | OtherNames = Biogas<br>Carbon tetrahydride<br>Hydrogen carbide<br>Marsh gas<br>Methyl hydride<br>Natural gas | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = Liquid methane with lots of condensation by ChemicalForce.jpg |
| − | | ImageSize = | + | | ImageSize = 300 |
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| + | | ImageCaption = Liquid methane, being poured in a dish, while causing lots of moisture condensation | ||
| ImageFile1 = | | ImageFile1 = | ||
| ImageSize1 = | | ImageSize1 = | ||
| Line 44: | Line 45: | ||
| 3DMet = | | 3DMet = | ||
| Abbreviations = | | Abbreviations = | ||
| − | | SMILES = | + | | SMILES = C |
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| Line 112: | Line 113: | ||
}} | }} | ||
}} | }} | ||
| − | '''Methane''' is a chemical compound with the chemical formula '''CH<sub>4</sub>'''. It is the simplest alkane and the main component of natural gas. | + | '''Methane''' is a chemical compound with the chemical formula '''CH<sub>4</sub>'''. It is the simplest alkane and organic compound, as well as the main component of natural gas. |
Methane is also partly responsible for climate change, as it is a very potent greenhouse gas. | Methane is also partly responsible for climate change, as it is a very potent greenhouse gas. | ||
| Line 122: | Line 123: | ||
: CH<sub>4</sub> + 2 O<sub>2</sub> → CO<sub>2</sub> + 2 H<sub>2</sub>O | : CH<sub>4</sub> + 2 O<sub>2</sub> → CO<sub>2</sub> + 2 H<sub>2</sub>O | ||
| − | Minute amounts of [[formaldehyde]] | + | Minute amounts of [[formaldehyde]] are also produced as a side product if excess methane is used. |
| − | Methane is very slightly basic. | + | Methane is very slightly basic, however, in general it's unreactive towards most reagents at standard conditions. |
===Physical=== | ===Physical=== | ||
| Line 130: | Line 131: | ||
==Availability== | ==Availability== | ||
| − | Methane is sold in compressed gas cylinders, mixed with small amounts of tert-butylthiol, which gives it the characteristic "gas" smell. | + | Methane is sold in compressed gas cylinders, mixed with small amounts of tert-butylthiol, which gives it the characteristic "gas" smell. The thiol can be removed by bubbling the gas through a scrubber. This experiment should only be performed in a well-ventilated area, as pure methane is odorless and in case of a leak, it may become an explosive hazard. |
| − | Natural gas can also serve as methane substitute in reactions that do not require high reagent grades. Important: most household gas cylinders are not natural gas but rather propane, butane or a mix of both. | + | Natural gas can also serve as a methane substitute in reactions that do not require high reagent grades. Important: most household gas cylinders are not natural gas but rather [[propane]], [[butane]] or a mix of both. |
==Preparation== | ==Preparation== | ||
Methane can be prepared from the decarboxylation of [[sodium acetate]] with [[sodium hydroxide]]. | Methane can be prepared from the decarboxylation of [[sodium acetate]] with [[sodium hydroxide]]. | ||
| − | :CH<sub>3</sub>COONa + NaOH → CH<sub>4</sub> + Na<sub>2</sub>CO<sub>3</sub> | + | : CH<sub>3</sub>COONa + NaOH → CH<sub>4</sub> + Na<sub>2</sub>CO<sub>3</sub> |
| − | [[Ethane]] appears as side product. | + | [[Ethane]] appears as a side product. |
Another way to prepare methane is via reduction of [[methanol]] with [[hydroiodic acid]], in the presence of a platinum catalyst.<ref>http://www.sciencedirect.com/science/article/pii/0360319987900140</ref> | Another way to prepare methane is via reduction of [[methanol]] with [[hydroiodic acid]], in the presence of a platinum catalyst.<ref>http://www.sciencedirect.com/science/article/pii/0360319987900140</ref> | ||
Another simple way is to add water to [[aluminium carbide]]. Aluminium carbide can be produced by reducing powdered [[aluminium oxide|alumina]] with [[carbon]] at high temperatures. | Another simple way is to add water to [[aluminium carbide]]. Aluminium carbide can be produced by reducing powdered [[aluminium oxide|alumina]] with [[carbon]] at high temperatures. | ||
| − | :Al<sub>4</sub>C<sub>3</sub> + 12 H<sub>2</sub>O → 3 CH<sub>4</sub> + 4 Al(OH)<sub>3</sub> | + | : Al<sub>4</sub>C<sub>3</sub> + 12 H<sub>2</sub>O → 3 CH<sub>4</sub> + 4 Al(OH)<sub>3</sub> |
This reaction works better at high temperatures and low oxygen atmosphere. | This reaction works better at high temperatures and low oxygen atmosphere. | ||
| − | The best way to make methane is from the decomposition of organic matter, in a reactor called digester, which can be anything from a plastic barrel to corrosion resistant metal tanks. Inside the tank, organic matter or manure (herbivores only!) is mixed with water and | + | The best way to make methane is from the decomposition of organic matter, in a reactor called digester, which can be anything from a plastic barrel to corrosion-resistant metal tanks. Inside the tank, organic matter or manure (herbivores only!) is mixed with water and left to decompose over a period of weeks to months. The resulting gas, called "biogas", is transferred in another container, usually filled with water, from where it's purified. Raw biogas generally contains 50-75% methane, 25-50% [[carbon dioxide]], nitrogen (<10%), with the rest being [[hydrogen sulfide]] (~3%), water vapor (1%), hydrogen (~1%), oxygen (0.5%) and sometimes traces of siloxanes, phosphine or amines such as ammonia. The impurities are removed by scrubbing the biogas in either an alkaline solution or in special adsorption towers. The purified methane is dried and, since this process takes a long time, can be compressed in gas tanks. NEVER store compressed methane in oxygen cylinders! |
==Projects== | ==Projects== | ||
Latest revision as of 16:35, 22 July 2023
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
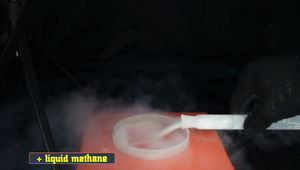 Liquid methane, being poured in a dish, while causing lots of moisture condensation
| |
| Names | |
|---|---|
| IUPAC name
Methane
| |
| Other names
Biogas
Carbon tetrahydride Hydrogen carbide Marsh gas Methyl hydride Natural gas | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| CH4 | |
| Molar mass | 16.04 g/mol |
| Appearance | Colorless gas |
| Odor | Odorless |
| Density | 0.656 g/L (gas, 25 °C, 1 atm) 0.716 g/L (gas, 0 °C, 1 atm) 0.42262 g/cm3 (liquid, −162 °C) |
| Melting point | −182.5 °C (−296.5 °F; 90.6 K) |
| Boiling point | −161.49 °C (−258.68 °F; 111.66 K) |
| 22.7 mg/L | |
| Solubility | Soluble in acetone, benzene, diethyl ether, ethanol, methanol, toluene, xylene |
| Solubility in diethyl ether | 65 ml/100 ml (20 °C) |
| Solubility in ethanol | 36 ml/100 ml (20 °C) |
| Vapor pressure | 4.66·105 mmHg at 25 °C |
| Thermochemistry | |
| Std molar
entropy (S |
186.25 J·K−1·mol−1 |
| Std enthalpy of
formation (ΔfH |
−74.87 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | −188 °C (−306.4 °F; 85.1 K) |
| Related compounds | |
| Related compounds
|
Ethane |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methane is a chemical compound with the chemical formula CH4. It is the simplest alkane and organic compound, as well as the main component of natural gas.
Methane is also partly responsible for climate change, as it is a very potent greenhouse gas.
Contents
Properties
Chemical
Methane will burn in air to produce carbon dioxide and water vapor.
- CH4 + 2 O2 → CO2 + 2 H2O
Minute amounts of formaldehyde are also produced as a side product if excess methane is used.
Methane is very slightly basic, however, in general it's unreactive towards most reagents at standard conditions.
Physical
Methane is a gas at standard conditions, lighter than air. It is colorless, odorless (the smell of flatulence comes from impurities in the source that give a warning of a leak), insoluble in water, but soluble in organic solvents. Methane has a melting point of -182.5 °C and a boiling point of −161 °C.
Availability
Methane is sold in compressed gas cylinders, mixed with small amounts of tert-butylthiol, which gives it the characteristic "gas" smell. The thiol can be removed by bubbling the gas through a scrubber. This experiment should only be performed in a well-ventilated area, as pure methane is odorless and in case of a leak, it may become an explosive hazard.
Natural gas can also serve as a methane substitute in reactions that do not require high reagent grades. Important: most household gas cylinders are not natural gas but rather propane, butane or a mix of both.
Preparation
Methane can be prepared from the decarboxylation of sodium acetate with sodium hydroxide.
- CH3COONa + NaOH → CH4 + Na2CO3
Ethane appears as a side product.
Another way to prepare methane is via reduction of methanol with hydroiodic acid, in the presence of a platinum catalyst.[1]
Another simple way is to add water to aluminium carbide. Aluminium carbide can be produced by reducing powdered alumina with carbon at high temperatures.
- Al4C3 + 12 H2O → 3 CH4 + 4 Al(OH)3
This reaction works better at high temperatures and low oxygen atmosphere.
The best way to make methane is from the decomposition of organic matter, in a reactor called digester, which can be anything from a plastic barrel to corrosion-resistant metal tanks. Inside the tank, organic matter or manure (herbivores only!) is mixed with water and left to decompose over a period of weeks to months. The resulting gas, called "biogas", is transferred in another container, usually filled with water, from where it's purified. Raw biogas generally contains 50-75% methane, 25-50% carbon dioxide, nitrogen (<10%), with the rest being hydrogen sulfide (~3%), water vapor (1%), hydrogen (~1%), oxygen (0.5%) and sometimes traces of siloxanes, phosphine or amines such as ammonia. The impurities are removed by scrubbing the biogas in either an alkaline solution or in special adsorption towers. The purified methane is dried and, since this process takes a long time, can be compressed in gas tanks. NEVER store compressed methane in oxygen cylinders!
Projects
- Methyl chloride synthesis
- Methanol synthesis
- Methane soap bubbles
Handling
Safety
Methane is not toxic, but it's extremely flammable and can form explosive mixtures with air, at concentrations between 4.4-17%. Methane is also an asphyxiant and may displace oxygen in an enclosed space. However, because it's lighter than air, it doesn't tend to accumulate.
Storage
Methane gas cylinders should be stored in cold places and away from any strong oxidizing or corrosive source. Valves should always be checked for leaks.
Disposal
Methane is a potent gas involved in global warming, so it's recommended to burn it when possible, instead of letting it rise in the atmosphere.
References
Relevant Sciencemadness threads
- Article stubs
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Hydrocarbons
- Alkanes
- Gases
- Biologically-derived compounds