Difference between revisions of "Formic acid"
| (4 intermediate revisions by 2 users not shown) | |||
| Line 15: | Line 15: | ||
| ImageAlt1 = | | ImageAlt1 = | ||
| ImageName1 = | | ImageName1 = | ||
| + | | ImageCaption1 = Formic acid sample and original bottle | ||
| ImageFile2 = Formic acid.svg.png | | ImageFile2 = Formic acid.svg.png | ||
| ImageSize2 = 250 | | ImageSize2 = 250 | ||
| ImageAlt2 = | | ImageAlt2 = | ||
| ImageName2 = | | ImageName2 = | ||
| + | | ImageCaption2 = Structure of formic acid | ||
| ImageFile3 = | | ImageFile3 = | ||
| ImageSize3 = | | ImageSize3 = | ||
| Line 118: | Line 120: | ||
As with other carboxylic acids, formic acid is easily [[esterification|esterified]] with primary alcohols, often forming pleasant-smelling compounds such as [[methyl formate]]. Being a strong acid, esterifications with formic acid generally don't require an acid catalyst. | As with other carboxylic acids, formic acid is easily [[esterification|esterified]] with primary alcohols, often forming pleasant-smelling compounds such as [[methyl formate]]. Being a strong acid, esterifications with formic acid generally don't require an acid catalyst. | ||
| − | In organic chemistry, formic acid is used to introduce a | + | In organic chemistry, formic acid is used to introduce a formyl group. |
===Physical=== | ===Physical=== | ||
| Line 131: | Line 133: | ||
==Preparation== | ==Preparation== | ||
| − | Aqueous formic acid can be distilled from a mixture of anhydrous [[glycerol]] and [[oxalic acid]], producing carbon dioxide as a byproduct. It can also be obtained by acidifying [[sodium formate]] with '''dilute''' [[sulfuric acid]] or [[phosphoric acid]], producing formic acid in solution that can then be distilled over. Using concentrated sulfuric acid or heating too much will produce [[carbon monoxide]] and potentially create a very dangerous situation. | + | Aqueous formic acid can be distilled from a mixture of anhydrous [[glycerol]] and [[oxalic acid]], producing carbon dioxide as a byproduct.<ref>NurdRage, ''Make Formic Acid'', [https://www.youtube.com/watch?v=ceL-I0azPH8 https://www.youtube.com/watch?v=ceL-I0azPH8]</ref> It can also be obtained by acidifying [[sodium formate]] with '''dilute''' [[sulfuric acid]] or [[phosphoric acid]], producing formic acid in solution that can then be distilled over. Using concentrated sulfuric acid or heating too much will produce [[carbon monoxide]] and potentially create a very dangerous situation. |
==Projects== | ==Projects== | ||
| Line 176: | Line 178: | ||
[[Category:DEA SS List]] | [[Category:DEA SS List]] | ||
[[Category:Irritants]] | [[Category:Irritants]] | ||
| + | [[Category:Reducing agents]] | ||
| + | [[Category:Essential reagents]] | ||
Latest revision as of 15:05, 18 November 2023
 Formic acid sample and original bottle
| |
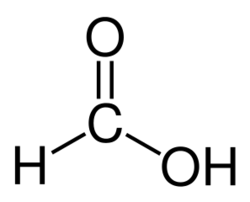 Structure of formic acid
| |
| Names | |
|---|---|
| IUPAC name
Methanoic acid
| |
| Preferred IUPAC name
Methanoic acid | |
| Systematic IUPAC name
Methanoic acid | |
| Other names
Aminic acid
Bilorin Carbonous acid Formylic acid Hydrogen carboxylic acid Hydroxy(oxo)methane Metacarbonoic acid Oxocarbinic acid Oxomethanol | |
| Properties | |
| CH2O2 HCOOH | |
| Molar mass | 46.03 g/mol |
| Appearance | Colorless liquid |
| Odor | Pungent, ants-like |
| Density | 1.220 g/cm3 (20 °C) |
| Melting point | 8.4 °C (47.1 °F; 281.5 K) |
| Boiling point | 100.8 °C (213.4 °F; 373.9 K) |
| Miscible | |
| Solubility | Reacts with amines, bases Miscible with acetone, diethyl ether, ethanol, ethyl acetate, glycerol, methanol Partially soluble in benzene, toluene, xylene |
| Vapor pressure | 35 mmHg (20 °C) |
| Acidity (pKa) | 3.77 |
| Thermochemistry | |
| Std molar
entropy (S |
131.8 J·mol-1·K-1 |
| Std enthalpy of
formation (ΔfH |
−425.0 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich (85% solution) |
| Flash point | 69 °C (156 °F; 342 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
700 mg/kg (mouse, oral) 1,100 mg/kg (rat, oral) 4,000 mg/kg (dog, oral) |
| LC50 (Median concentration)
|
7,853 ppm (rat, 15 min) 3,246 ppm (mouse, 15 min) |
| Related compounds | |
| Related compounds
|
Acetic acid Oxalic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Formic acid or methanoic acid is an organic compound with chemical formula HCOOH and the simplest carboxylic acid.
Contents
Properties
Chemical
Formic acid is readily dehydrated by concentrated sulfuric acid to carbon monoxide, and is often used in the lab as a convenient and store-able source of the gas.
- HCOOH + H2SO4 → CO + H2O + H2SO4
As with other carboxylic acids, formic acid is easily esterified with primary alcohols, often forming pleasant-smelling compounds such as methyl formate. Being a strong acid, esterifications with formic acid generally don't require an acid catalyst.
In organic chemistry, formic acid is used to introduce a formyl group.
Physical
Formic acid is a clear liquid with a highly irritating pungent odor, and is responsible for the painful sensation of many ant stings. Its boiling point is nearly the same as water (100.8 ˚C), though it forms an azeotrope of 22.4% formic acid with water that boils instead at 107.3 ˚C.
Since formic acid slowly decomposes when boiled, distillation must be done at low temperature and in near vacuum (25 °C at 40 mm Hg).[1]
Availability
While not typically found as a consumer product, formic acid can be bought online for relatively low prices, typically mixed with about 5-10% water. A good supplier is Duda Diesel.
Formic acid can also be purchased at beekeeping stores, as 60-85% concentration. It is used for the treatment of Varroasis.
Preparation
Aqueous formic acid can be distilled from a mixture of anhydrous glycerol and oxalic acid, producing carbon dioxide as a byproduct.[2] It can also be obtained by acidifying sodium formate with dilute sulfuric acid or phosphoric acid, producing formic acid in solution that can then be distilled over. Using concentrated sulfuric acid or heating too much will produce carbon monoxide and potentially create a very dangerous situation.
Projects
- Make formate esters
- Generate carbon monoxide for reactions
- Formic anhydride synthesis (stable only as solution in diethyl ether)
Handling
Safety
Concentrated solutions of formic acid are corrosive to human skin, as well as nose, mouth and eyes and also slowly decompose to form water and carbon monoxide, which can cause an explosion from pressure buildup in a sealed container. Adding a dehydrating agent will also generate large amounts of carbon monoxide, a deadly gas that is impossible to detect with human senses. Formic acid is not inherently very toxic if ingested, though long exposure to it by any means can cause chronic bodily effects. It is especially important that formic acid is kept away from the eyes, as it readily damages the optic nerve and can cause permanent blindness.
Storage
Formic acid should be stored in closed bottles, away from any heat source. Keep it away from dwelling areas, as it slowly gives off carbon monoxide over time.
Disposal
Formic acid should be neutralized before disposal. This can be done by diluting the acid, then slowly adding it in an aqueous solution of a base. like sodium carbonate or sodium bicarbonate.
References
- ↑ Purification of Laboratory Chemicals (Fifth Edition), Wilfred L.F. Armarego and Christina Li Lin Chai, 2003
- ↑ NurdRage, Make Formic Acid, https://www.youtube.com/watch?v=ceL-I0azPH8