Difference between revisions of "Methyl iodide"
| Line 114: | Line 114: | ||
===Chemical=== | ===Chemical=== | ||
Methyl iodide will hydrolyze in the presence of an alkali to [[methanol]] and iodide: | Methyl iodide will hydrolyze in the presence of an alkali to [[methanol]] and iodide: | ||
| + | |||
:CH<sub>3</sub>I + MOH → CH<sub>3</sub>OH + MI | :CH<sub>3</sub>I + MOH → CH<sub>3</sub>OH + MI | ||
| + | |||
| + | Methyl iodide will react with a nitrite salt, yielding [[nitromethane]] if [[sodium nitrite|sodium]] or [[potassium nitrite]] are used, with methyl nitrite as minor product. If [[silver nitrite]] is used, methyl nitrite will be the majority product, due to the fact that the silver ion in solution has a stabilizing effect on the formation of carbocation intermediates, increasing the percent yield of methyl nitrite. If [[nitrogen dioxide]] is bubbled in methyl iodide, methyl nitrite will be the sole product obtained. | ||
| + | |||
| + | : CH<sub>3</sub>I + NO<sub>2</sub> → CH<sub>3</sub>NO<sub>2</sub> + ½ I<sub>2</sub> | ||
===Physical=== | ===Physical=== | ||
| Line 125: | Line 130: | ||
==Preparation== | ==Preparation== | ||
| − | Methyl iodide can be prepared by reacting [[methanol]] with [[hydroiodic acid]] or [[hydrogen iodide]]. While the methanol does not need to be | + | Methyl iodide can be prepared by reacting [[methanol]] with [[hydroiodic acid]] or [[hydrogen iodide]]. While the methanol does not need to be anhydrous, excess water may lower the yield.<ref>http://www.orgsyn.org/demo.aspx?prep=CV2P0399</ref> |
[[Phosphorus triiodide]] can also be used. This can be made in situ by adding iodine and then adding red [[phosphorus]] in dry methanol and refluxing the mixture. | [[Phosphorus triiodide]] can also be used. This can be made in situ by adding iodine and then adding red [[phosphorus]] in dry methanol and refluxing the mixture. | ||
| Line 143: | Line 148: | ||
===Disposal=== | ===Disposal=== | ||
| − | Methyl iodide can be hydrolyzed to methanol with sodium hydroxide. | + | Methyl iodide can be hydrolyzed to methanol with [[sodium hydroxide]], which can be safely burned, while sodium iodide can be recycled. |
==References== | ==References== | ||
| Line 149: | Line 154: | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
*[http://www.sciencemadness.org/talk/viewthread.php?tid=12475 Preparation of Iodomethane] | *[http://www.sciencemadness.org/talk/viewthread.php?tid=12475 Preparation of Iodomethane] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=18849 Methyl Iodide Synthesis ?] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
Revision as of 15:33, 10 September 2018
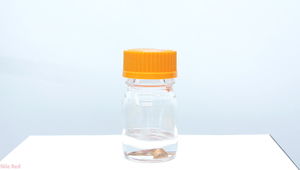 Freshly prepared methyl iodide, stored over copper
| |
| Names | |
|---|---|
| IUPAC name
Iodomethane
| |
| Other names
Methyl iodine
Monoiodomethane | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| CH3I | |
| Molar mass | 141.94 g/mol |
| Appearance | Colorless liquid Brownish liquid (old sample) |
| Odor | Pungent, ether-like |
| Density | 2.28 g/cm3 (at 20 °C) |
| Melting point | −66.5 °C (−87.7 °F; 206.7 K) |
| Boiling point | 42.5 °C (108.5 °F; 315.6 K) |
| 1.4 g/100 ml (at 20 °C) | |
| Solubility | Miscible with acetone, alcohols, carbon tetrachloride, chloroform, diethyl ether |
| Vapor pressure | 54.4 kPa (at 20 °C) |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
−14.1–−13.1 kJ/mol |
| Hazards | |
| Safety data sheet | ScienceLab |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
76 mg/kg (oral, rat) 800 mg/kg (dermal, guinea pig) |
| LC50 (Median concentration)
|
1550 ppm (rat, 30 min) 860 ppm (mouse, 57 min) 220 ppm (rat, 4 hr) |
| Related compounds | |
| Related compounds
|
Iodine Iodoform Ethyl iodide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methyl iodide or iodomethane is an organic chemical compound, which appears as a dense liquid, insoluble in water, mainly used as a methylating agent. It has the chemical formula CH3I, sometimes written as MeI.
Contents
Properties
Chemical
Methyl iodide will hydrolyze in the presence of an alkali to methanol and iodide:
- CH3I + MOH → CH3OH + MI
Methyl iodide will react with a nitrite salt, yielding nitromethane if sodium or potassium nitrite are used, with methyl nitrite as minor product. If silver nitrite is used, methyl nitrite will be the majority product, due to the fact that the silver ion in solution has a stabilizing effect on the formation of carbocation intermediates, increasing the percent yield of methyl nitrite. If nitrogen dioxide is bubbled in methyl iodide, methyl nitrite will be the sole product obtained.
- CH3I + NO2 → CH3NO2 + ½ I2
Physical
Methyl iodide is a dense, colorless (though old samples have a brownish color) liquid, immiscible in water, but miscible with many solvents such as diethyl ether, ethanol, methanol and soluble in acetone and carbon tetrachloride. Methyl iodide has a strong and pungent ether-like odor.
Availability
Methyl iodide is sold by various chemical suppliers, though it's difficult to acquire. It can sometimes be found on eBay.
Methyl iodide used to be available as a pesticide up until 2012, when it was removed due to its carcinogenic effects.
Preparation
Methyl iodide can be prepared by reacting methanol with hydroiodic acid or hydrogen iodide. While the methanol does not need to be anhydrous, excess water may lower the yield.[1]
Phosphorus triiodide can also be used. This can be made in situ by adding iodine and then adding red phosphorus in dry methanol and refluxing the mixture.
Projects
- Make esters
- Make toluene
Handling
Safety
Iodomethane is toxic and carcinogenic.
Methyl iodide MUST be kept away from heavy metals, such as mercury, lead, cadmium, arsenic or thallium, as this will generate extremely toxic heavy methyl iodides, which are more toxic than their corresponding metals. It's ABSOLUTELY CRUCIAL to avoid contact with mercury amalgam, as this will yield the volatile compound dimethylmercury, one of the most deadly contact poisons known, second only to nerve agents, which readily penetrates butyl, latex, neoprene and PVC protective equipment.
Storage
Methyl iodide must be stored in amber bottles, in dark well ventilated places, away from light. Copper or silver wire can be added as a stabilizer.
Disposal
Methyl iodide can be hydrolyzed to methanol with sodium hydroxide, which can be safely burned, while sodium iodide can be recycled.
References
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Organoiodine compounds
- Haloalkanes
- Solvents
- Volatile chemicals
- Methylating agents
- Carcinogenic
- Liquids