Difference between revisions of "Guanidine"
(→Preparation) |
|||
| Line 7: | Line 7: | ||
| OtherNames = Aminomethanamidine<br>Carbamamidine<br>Carbamidine<br>Gdn<br>Guanidin<br>Imidourea | | OtherNames = Aminomethanamidine<br>Carbamamidine<br>Carbamidine<br>Gdn<br>Guanidin<br>Imidourea | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = Guanidine base structure.png |
| ImageSize = | | ImageSize = | ||
| ImageAlt = | | ImageAlt = | ||
| Line 125: | Line 125: | ||
==Preparation== | ==Preparation== | ||
| − | Free base guanidine can be prepared from | + | Free base guanidine can be prepared from guanidinium salts by adding [[sodium hydroxide]] to said guanidinium salt, then recrystallizing it from the solvent. |
To obtain water-free freebase guanidine, a simple method is to add a guanidinium salt, like guanidinium chloride or sulfate to a methanolic solution of [[sodium methoxide]] (which can be easily made by adding [[sodium]] metal to dried [[methanol]]). The sodium salt will precipitate out of the solution, while the freebase guanidine will remain in solution. | To obtain water-free freebase guanidine, a simple method is to add a guanidinium salt, like guanidinium chloride or sulfate to a methanolic solution of [[sodium methoxide]] (which can be easily made by adding [[sodium]] metal to dried [[methanol]]). The sodium salt will precipitate out of the solution, while the freebase guanidine will remain in solution. | ||
Revision as of 14:14, 31 December 2017
| |
| Names | |
|---|---|
| IUPAC names
Guanidine
Iminomethanediamine | |
| Other names
Aminomethanamidine
Carbamamidine Carbamidine Gdn Guanidin Imidourea | |
| Properties | |
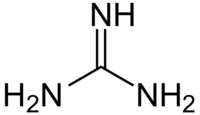
| CH5N3 HNC(NH2)2 | |
| Molar mass | 59.07 g/mol |
| Appearance | Hygroscopic colorless solid |
| Odor | Odorless |
| Density | 1.6 g/cm3 |
| Melting point | 50 °C (122 °F; 323 K) |
| Boiling point | Decomposes |
| 0.184 g/100 ml (20 °C) | |
| Solubility | Soluble in polar solvents |
| Vapor pressure | 2.2 mmHg at 25 °C |
| Acidity (pKa) | 12.5 |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
−57 – −55 kJ/mol |
| Hazards | |
| Safety data sheet | Fluorochem |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
475 mg/kg (rat, oral)[1] |
| Related compounds | |
| Related compounds
|
Ammonia Hydrazine Urea |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Guanidine is an organic compound, a base with the formula HNC(NH2)2. Guanidine is sometimes shortened to Gdn.
Contents
Properties
Chemical
As a base, guanidine will react with acids to form salts. By protanating guanidine, the guanidinium ion is formed.
- HNC(NH2)2 + HCl → C(NH2)3+Cl-
Physical
Guanidine is a solid white hygroscopic compound, slightly soluble in water.
Availability
Free base guanidine is difficult to find. Fluorochem and Oakwood Chemical are one of the few suppliers that sell the free base compound.
Guanidinium salts, of the other hand, can be purchased from many chemical suppliers. To obtain the free base, simply react the salt with a stronger base then extract the resulting guanidine.
Preparation
Free base guanidine can be prepared from guanidinium salts by adding sodium hydroxide to said guanidinium salt, then recrystallizing it from the solvent.
To obtain water-free freebase guanidine, a simple method is to add a guanidinium salt, like guanidinium chloride or sulfate to a methanolic solution of sodium methoxide (which can be easily made by adding sodium metal to dried methanol). The sodium salt will precipitate out of the solution, while the freebase guanidine will remain in solution.
- C(NH2)3Cl + CH3ONa → HNC(NH2)2 + CH3OH + NaCl
For the preparation of guanidinium salts, check the page for each compound.
Guanidine can also be obtained from oxidative degradation of guanine, isolated from Peruvian guano (hence its name). Guano fertilizer can be bought from many hardware and gardening stores.
Projects
- Make rocket fuel
- Make guanidinium salts (guanidinium carbonate, guanidinium chloride, guanidinium nitrate, guanidinium perchlorate, guanidinium sulfate)
Handling
Safety
Guanidine and its salts aren't volatile or very toxic and don't require special handling.
Storage
Guanidine free base should be kept in closed bottles, in a dry place.
Disposal
No special disposal is required. Some guanidinium salts are even used as fertilizer.