Difference between revisions of "Methyl formate"
(→Preparation) |
|||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Chembox |
| + | | Name = Methyl formate | ||
| + | | Reference = | ||
| + | | IUPACName = Methyl formate | ||
| + | | PIN = Methyl formate | ||
| + | | SystematicName = | ||
| + | | OtherNames = R-611 | ||
| + | <!-- Images --> | ||
| + | | ImageFile = | ||
| + | | ImageSize = | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageFile1 = | ||
| + | | ImageSize1 = | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = Colorless volatile liquid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 32 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = | ||
| + | | Density = 0.987 g/cm<sup>3</sup> (15 °C)<br>0.98 g/cm<sup>3</sup> (20 °C) | ||
| + | | Formula = C<sub>2</sub>H<sub>4</sub>O<sub>2</sub> | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| + | | MolarMass = 60.05 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = −100 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | Odor = Pleasant, lemonade-like | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = 30% (at 20 °C) | ||
| + | | SolubleOther = Miscible with [[acetone]], [[chloroform]], [[ethanol]], [[methanol]] | ||
| + | | Solvent = | ||
| + | | VaporPressure = 476 mmHg (20 °C) | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = | ||
| + | | DeltaHf = | ||
| + | | Entropy = | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = | ||
| + | | ExploLimits = 4.5%-23% | ||
| + | | ExternalMSDS = [https://www.docdroid.net/GYK0OEu/methyl-formate-sa.pdf.html Simga-Aldrich] | ||
| + | | FlashPt = −19 °C | ||
| + | | LD50 = | ||
| + | | LC50 = | ||
| + | | MainHazards = Irritant<br>Flammable<br>Volatile | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Ethyl formate]]<br>[[Methyl acetate]] | ||
| + | }} | ||
| + | }} | ||
[[File:Methyl formate structure.png|thumb|250x200px]] | [[File:Methyl formate structure.png|thumb|250x200px]] | ||
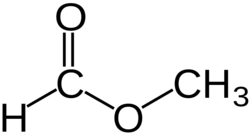
'''Methyl formate''' or '''methyl methanoate''', is the methyl ester of [[formic acid]], the simplest ester. | '''Methyl formate''' or '''methyl methanoate''', is the methyl ester of [[formic acid]], the simplest ester. | ||
| Line 31: | Line 139: | ||
===Storage=== | ===Storage=== | ||
| − | Due to its low boiling point, | + | Due to its low boiling point, methyl formate should be stored in closed bottles, away from any source of heat or light, preferable in a fridge. This is mandatory during hot summers. |
===Disposal=== | ===Disposal=== | ||
Revision as of 19:22, 22 February 2017
| Names | |
|---|---|
| IUPAC name
Methyl formate
| |
| Preferred IUPAC name
Methyl formate | |
| Other names
R-611
| |
| Properties | |
| C2H4O2 | |
| Molar mass | 60.05 g/mol |
| Appearance | Colorless volatile liquid |
| Odor | Pleasant, lemonade-like |
| Density | 0.987 g/cm3 (15 °C) 0.98 g/cm3 (20 °C) |
| Melting point | −100 °C (−148 °F; 173 K) |
| Boiling point | 32 °C (90 °F; 305 K) |
| 30% (at 20 °C) | |
| Solubility | Miscible with acetone, chloroform, ethanol, methanol |
| Vapor pressure | 476 mmHg (20 °C) |
| Hazards | |
| Safety data sheet | Simga-Aldrich |
| Flash point | −19 °C |
| Related compounds | |
| Related compounds
|
Ethyl formate Methyl acetate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methyl formate or methyl methanoate, is the methyl ester of formic acid, the simplest ester.
Contents
Properties
Chemical
Methyl formate can be hydrolyzed to methanol and formic acid.
Physical
Methyl formate is a colorless organic liquid, with an ethereal odor, low surface tension and high vapor pressure. Its melting point is at −100 °C and boils at 32 °C. It has a density of 0.97 g/cm3. It is soluble in water (30g/100 ml) and other solvents, such as ethyl ether, acetone. Its flash point is -19°C and its auto-ignition temperature is 449°C.
Availability
Methyl formate is available from chemical suppliers.
Preparation
Methyl formate can be synthesized by reacting anhydrous formic acid with dry methanol, over a strong desiccant, such as calcium chloride. Due to its low boiling point, methyl formate can be extracted via fractional distillation.
Industrially, it is prepared by via carbonylation of methanol, using sodium methoxide as a catalyst and pyridine as a temperature promoter, in an extremely dry medium. The smallest traces of water can disrupt the reaction.
- CH3OH + CO → HCOOCH3[1]
This route however is uneconomical for the amateur chemist, thus the first method is cheaper and more accessible.
Projects
- Formamide synthesis
- Organic extractions
Handling
Safety
Methyl formate vapors may irritate lungs, so it's best to work in a well ventilated area. Since its boiling point is lower than the human body temperature, samples of methyl formate should not be kept too long in hand.
Storage
Due to its low boiling point, methyl formate should be stored in closed bottles, away from any source of heat or light, preferable in a fridge. This is mandatory during hot summers.
Disposal
Methyl formate can be safely burned.