Difference between revisions of "Acetic anhydride"
(→Preparation) |
|||
| (9 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[ | + | {{Chembox |
| + | | Name = Acetic anhydride | ||
| + | | Reference = | ||
| + | | IUPACName = Acetic anhydride | ||
| + | | PIN = Acetic anhydride | ||
| + | | SystematicName = Ethanoic anhydride | ||
| + | | OtherNames = Ethanoyl ethanoate<br>Acetic acid anhydride<br>Acetyl acetate<br>Acetyl oxide<br>Acetic oxide | ||
| + | <!-- Images --> | ||
| + | | ImageFile = Acetic anhydride.png | ||
| + | | ImageSize = 250 | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageCaption = Acetic anhydride structure | ||
| + | | ImageFile1 = | ||
| + | | ImageSize1 = | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = Colorless liquid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 139.8 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = | ||
| + | | Density = 1.082 g/cm<sup>3</sup> | ||
| + | | Formula = C<sub>4</sub>H<sub>6</sub>O<sub>3</sub> | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| + | | MolarMass = 102.09 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = −73.1 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | Odor = Acetic | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = Slowly hydrolyzes | ||
| + | | SolubleOther = Miscible with [[acetic acid]], [[diethyl ether]], [[ethanol]], [[ethyl acetate]] | ||
| + | | Solvent = | ||
| + | | VaporPressure = 4 mmHg (20 °C) | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = | ||
| + | | DeltaHf = | ||
| + | | Entropy = | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = 316 °C (601 °F; 589 K) | ||
| + | | ExploLimits = 2.7–10.3% | ||
| + | | ExternalMSDS = [https://www.docdroid.net/AOc4ly6/acetic-anhydride-sa.pdf.html Sigma-Aldrich] | ||
| + | | FlashPt = 49 °C (120 °F; 322 K) | ||
| + | | LD50 = | ||
| + | | LC50 = 1,000 ppm (rat, 4 hr) | ||
| + | | MainHazards = | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Acetic acid]]<br>[[Propionic anhydride]] | ||
| + | }} | ||
| + | }} | ||
'''Acetic anhydride''' is a colorless organic liquid with the formula ('''CH<sub>3</sub>CO)<sub>2</sub>O''' and the simplest example of a carboxylic acid anhydride (the simpler formic anhydride is too unstable to exist as pure substance). It is a very useful reagent in organic synthesis. | '''Acetic anhydride''' is a colorless organic liquid with the formula ('''CH<sub>3</sub>CO)<sub>2</sub>O''' and the simplest example of a carboxylic acid anhydride (the simpler formic anhydride is too unstable to exist as pure substance). It is a very useful reagent in organic synthesis. | ||
| Line 7: | Line 116: | ||
===Physical=== | ===Physical=== | ||
| − | Acetic anhydride is a colorless liquid with a density | + | Acetic anhydride is a colorless liquid with a density slightly greater than that of water. It is moderately flammable and, due to its volatility, smells strongly of acetic acid from reaction with water in the air. |
==Availability== | ==Availability== | ||
| Line 13: | Line 122: | ||
===Restrictions=== | ===Restrictions=== | ||
| − | Acetic anhydride is used illicitly in the drug trade to acetylate morphine to heroin. Because it is used for this purpose, it is classified in the US as a [[DEA | + | Acetic anhydride is used illicitly in the drug trade to acetylate morphine to heroin. Because it is used for this purpose, it is classified in the US as a [[DEA list of chemicals#List II chemicals|DEA List II]] chemical and as such is significantly harder to obtain. In Russia, it's a FSKN List I chemical and is impossible to obtain legally. This is also true for most countries. |
==Preparation== | ==Preparation== | ||
| − | Many amateurs have attempted to make acetic anhydride at home due to its tremendous usefulness. Only a few have succeeded, though a write-up of the preparation of acetic anhydride from [[sulfur]], [[bromine]], and anhydrous [[sodium acetate]] was reported by [http://www.sciencemadness.org/talk/viewthread.php?tid=15021 Magpie]. | + | Many amateurs have attempted to make acetic anhydride at home due to its tremendous usefulness in organic chemistry. Only a few have succeeded, though a write-up of the preparation of acetic anhydride from [[sulfur]], [[bromine]], and anhydrous [[sodium acetate]] was reported by [http://www.sciencemadness.org/talk/viewthread.php?tid=15021 Magpie]. |
| − | Another more accessible method involves the reaction of [[acetyl chloride]] and anhydrous [[sodium acetate]].<ref>https://www.erowid.org/archive/rhodium/chemistry/anhydrides.html</ref> | + | Another more accessible method involves the reaction of [[acetyl chloride]] and anhydrous [[sodium acetate]], though this is limited by the availability of acetyl chloride.<ref>https://www.erowid.org/archive/rhodium/chemistry/anhydrides.html</ref> |
| − | + | :CH<sub>3</sub>C(O)Cl + CH<sub>3</sub>COONa → (CH<sub>3</sub>CO)<sub>2</sub>O + NaCl | |
| + | |||
| + | [[Sulfur dichloride]] or [[disulfur dichloride|monochloride]] can also be used instead of acetyl chloride. However, the reaction tends to produce various organosulfur side products, which have a strong unpleasant smell.<ref>https://www.youtube.com/watch?v=OrZ5Oa9K1R0</ref> | ||
| + | |||
| + | A readily accessible route, but very dangerous, involves the reaction of [[ethenone]] with glacial acetic acid. | ||
| + | |||
| + | : CH<sub>3</sub>COOH + H<sub>2</sub>C=C=O → (CH<sub>3</sub>CO)<sub>2</sub>O | ||
| + | |||
| + | Ethenone can be easily produced by pyrolyzing acetone at high temperatures in an oxygen-free atmosphere, which is not very difficult to do, all you need is a reflux setup and an electric heating element. However, ethenone is an extremely toxic gas, with an LD50 close to that of [[hydrogen cyanide]] and [[phosgene]], and working with it carries the risk of severe injury or even death. | ||
| + | |||
| + | Other methods are discussed in the article about [[organic acid anhydrides]]. | ||
==Projects== | ==Projects== | ||
| Line 27: | Line 146: | ||
*Make cellulose acetate | *Make cellulose acetate | ||
*[[Acetophenone]] synthesis | *[[Acetophenone]] synthesis | ||
| + | *Make HMX (octogen) | ||
==Handling== | ==Handling== | ||
| Line 52: | Line 172: | ||
[[Category:DEA List II chemicals]] | [[Category:DEA List II chemicals]] | ||
[[Category:Liquids]] | [[Category:Liquids]] | ||
| + | [[Category:Irritants]] | ||
Latest revision as of 19:21, 12 February 2024
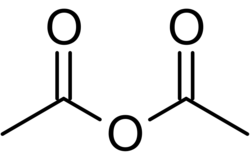 Acetic anhydride structure
| |
| Names | |
|---|---|
| IUPAC name
Acetic anhydride
| |
| Preferred IUPAC name
Acetic anhydride | |
| Systematic IUPAC name
Ethanoic anhydride | |
| Other names
Ethanoyl ethanoate
Acetic acid anhydride Acetyl acetate Acetyl oxide Acetic oxide | |
| Properties | |
| C4H6O3 | |
| Molar mass | 102.09 g/mol |
| Appearance | Colorless liquid |
| Odor | Acetic |
| Density | 1.082 g/cm3 |
| Melting point | −73.1 °C (−99.6 °F; 200.1 K) |
| Boiling point | 139.8 °C (283.6 °F; 412.9 K) |
| Slowly hydrolyzes | |
| Solubility | Miscible with acetic acid, diethyl ether, ethanol, ethyl acetate |
| Vapor pressure | 4 mmHg (20 °C) |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 49 °C (120 °F; 322 K) |
| Lethal dose or concentration (LD, LC): | |
| LC50 (Median concentration)
|
1,000 ppm (rat, 4 hr) |
| Related compounds | |
| Related compounds
|
Acetic acid Propionic anhydride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Acetic anhydride is a colorless organic liquid with the formula (CH3CO)2O and the simplest example of a carboxylic acid anhydride (the simpler formic anhydride is too unstable to exist as pure substance). It is a very useful reagent in organic synthesis.
Contents
Properties
Chemical
Acetic anhydride is most widely used as an agent for acetylation, the addition of an acetyl group to a compound, such as its reaction with ethanol to form ethyl acetate. Being a carboxylic acid anhydride, acetic anhydride will slowly decay in contact with water into acetic acid. This process, however, is slow enough for aqueous solutions to be made and used immediately.
Physical
Acetic anhydride is a colorless liquid with a density slightly greater than that of water. It is moderately flammable and, due to its volatility, smells strongly of acetic acid from reaction with water in the air.
Availability
Elemental Scientific sells acetic anhydride in 125ml and 500ml sizes. They, however, are one of the only reliable sellers who are selling this chemical in the US due to its status as DEA List II.
Restrictions
Acetic anhydride is used illicitly in the drug trade to acetylate morphine to heroin. Because it is used for this purpose, it is classified in the US as a DEA List II chemical and as such is significantly harder to obtain. In Russia, it's a FSKN List I chemical and is impossible to obtain legally. This is also true for most countries.
Preparation
Many amateurs have attempted to make acetic anhydride at home due to its tremendous usefulness in organic chemistry. Only a few have succeeded, though a write-up of the preparation of acetic anhydride from sulfur, bromine, and anhydrous sodium acetate was reported by Magpie.
Another more accessible method involves the reaction of acetyl chloride and anhydrous sodium acetate, though this is limited by the availability of acetyl chloride.[1]
- CH3C(O)Cl + CH3COONa → (CH3CO)2O + NaCl
Sulfur dichloride or monochloride can also be used instead of acetyl chloride. However, the reaction tends to produce various organosulfur side products, which have a strong unpleasant smell.[2]
A readily accessible route, but very dangerous, involves the reaction of ethenone with glacial acetic acid.
- CH3COOH + H2C=C=O → (CH3CO)2O
Ethenone can be easily produced by pyrolyzing acetone at high temperatures in an oxygen-free atmosphere, which is not very difficult to do, all you need is a reflux setup and an electric heating element. However, ethenone is an extremely toxic gas, with an LD50 close to that of hydrogen cyanide and phosgene, and working with it carries the risk of severe injury or even death.
Other methods are discussed in the article about organic acid anhydrides.
Projects
- Efficient acetate ester production
- Making aspirin from salicylic acid.
- Make cellulose acetate
- Acetophenone synthesis
- Make HMX (octogen)
Handling
Safety
Acetic anhydride, similar to glacial acetic acid, is highly acidic and corrosive, so proper protection must be worn when handling the compound. When mixed with hydrogen peroxide with the acetic anhydride in excess, a shock sensitive explosive, diacetyl peroxide, is formed.
Storage
Acetic anhydride should be stored in sealed bottles as it is sensitive to water. In addition, it should be stored away from any heat or flame source due to it's flammability .
Disposal
Acetic anhydride can be neutralized with sodium bicarbonate or any other base which is then safe to pour down the drain.
References
- ↑ https://www.erowid.org/archive/rhodium/chemistry/anhydrides.html
- ↑ https://www.youtube.com/watch?v=OrZ5Oa9K1R0