Difference between revisions of "Chloroacetic acid"
| (2 intermediate revisions by 2 users not shown) | |||
| Line 11: | Line 11: | ||
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| − | | ImageFile1 = | + | | ImageFile1 = Chloroacetic acid structural formula.png |
| − | | ImageSize1 = | + | | ImageSize1 = 180 |
| ImageAlt1 = | | ImageAlt1 = | ||
| ImageName1 = | | ImageName1 = | ||
| Line 43: | Line 43: | ||
| 3DMet = | | 3DMet = | ||
| Abbreviations = | | Abbreviations = | ||
| − | | SMILES = | + | | SMILES = c1ccc(c(c1)C(=O)O)O |
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| Line 53: | Line 53: | ||
| BoilingPt_notes = | | BoilingPt_notes = | ||
| Density = 1.58 g/cm<sup>3</sup> | | Density = 1.58 g/cm<sup>3</sup> | ||
| − | | Formula = C<sub>2</sub>H<sub>3</sub>ClO<sub>2</sub> | + | | Formula = C<sub>2</sub>H<sub>3</sub>ClO<sub>2</sub><br>ClCH<sub>2</sub>COOH |
| HenryConstant = | | HenryConstant = | ||
| LogP = 0.22 | | LogP = 0.22 | ||
| Line 65: | Line 65: | ||
| pKb = | | pKb = | ||
| Solubility = 85.8 g/100ml (25 °C) | | Solubility = 85.8 g/100ml (25 °C) | ||
| − | | SolubleOther = Soluble in [[acetone]], [[benzene]], [[chloroform]], [[diethyl ether]], [[ethanol]], [[methanol]]<br>Slightly soluble in [[carbon tetrachloride]], [[hexane]] | + | | SolubleOther = Reacts with alkalis, amines<br>Soluble in [[acetone]], [[benzene]], [[chloroform]], [[diethyl ether]], [[ethanol]], [[methanol]], [[tetrahydrofuran]], [[toluene]]<br>Slightly soluble in [[carbon tetrachloride]], [[hexane]] |
| Solvent = | | Solvent = | ||
| VaporPressure = 6.50·10<sup>-2</sup> mmHg at 25 °C | | VaporPressure = 6.50·10<sup>-2</sup> mmHg at 25 °C | ||
| Line 75: | Line 75: | ||
}} | }} | ||
| Section4 = {{Chembox Thermochemistry | | Section4 = {{Chembox Thermochemistry | ||
| − | | DeltaGf = | + | | DeltaGf = -368.5 kJ/mol |
| − | | DeltaHc = | + | | DeltaHc = 715.5 kJ/mol |
| DeltaHf = -490.1 kJ/mol | | DeltaHf = -490.1 kJ/mol | ||
| − | | Entropy = | + | | Entropy = 325.9 J·K<sup>-1</sup>·mol<sup>-1</sup> |
| HeatCapacity = 144.02 J·K<sup>-1</sup>·mol<sup>-1</sup> | | HeatCapacity = 144.02 J·K<sup>-1</sup>·mol<sup>-1</sup> | ||
}} | }} | ||
| Line 108: | Line 108: | ||
}} | }} | ||
}} | }} | ||
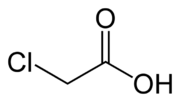
| − | '''Chloroacetic acid''' or '''monochloroacetic acid''' ('''MCA'''), is an organochlorine compound with the formula '''ClCH<sub>2</sub> | + | '''Chloroacetic acid''' or '''monochloroacetic acid''' ('''MCA'''), is an organochlorine compound with the formula '''ClCH<sub>2</sub>COOH'''. |
==Properties== | ==Properties== | ||
| Line 115: | Line 115: | ||
: ClCH<sub>2</sub>COOH + 2 NaOH → OH-CH<sub>2</sub>COONa + NaCl + H<sub>2</sub>O | : ClCH<sub>2</sub>COOH + 2 NaOH → OH-CH<sub>2</sub>COONa + NaCl + H<sub>2</sub>O | ||
| + | |||
| + | If the temperature is kept low, the hydrolysis of chloroacetic acid is limited. Since acid-base neutralization is exothermic, it's difficult to maintain a constant temperature. As such, glycolate-free chloroacetate salts can only be made safely by reacting the titular acid with metal carbonates or bicarbonates. | ||
| + | |||
| + | Reaction of sodium chloroacetate with [[sodium nitrite]] in aqueous solution will yield [[nitromethane]]:<ref>http://www.orgsyn.org/demo.aspx?prep=CV1P0401</ref> | ||
| + | |||
| + | : ClCH<sub>2</sub>COONa + NaNO<sub>2</sub> + H<sub>2</sub>O → CH<sub>3</sub>NO<sub>2</sub> + NaCl + NaHCO<sub>3</sub> | ||
| + | |||
| + | Chloroacetic acid will react with [[sodium thiosulfate]] to form a Bunte salt, which yields [[thioglycolic acid]] | ||
===Physical=== | ===Physical=== | ||
| Line 120: | Line 128: | ||
==Availability== | ==Availability== | ||
| − | Chloroacetic acid is | + | Chloroacetic acid is difficult to acquire in some countries, as it's very toxic. Its salts however, are much more accessible and safer to handle and can be bought online. |
==Preparation== | ==Preparation== | ||
Chloroacetic acid is prepared industrially via two routes. | Chloroacetic acid is prepared industrially via two routes. | ||
| − | The predominant method involves chlorination of acetic acid, with [[acetic anhydride]] as a catalyst, in the presence of UV light. [[Sulfur]] can also be used as catalyst<ref>http://www.prepchem.com/synthesis-of-chloroacetic-acid/</ref>, as can red [[phosphorus]]<ref>https://www.erowid.org/archive/rhodium/chemistry/chloroacetic.html</ref>. | + | The predominant method involves chlorination of glacial acetic acid, with [[acetic anhydride]] as a catalyst, in the presence of UV light. [[Sulfur]] can also be used as catalyst<ref>http://www.prepchem.com/synthesis-of-chloroacetic-acid/</ref>, as can red [[phosphorus]]<ref>https://www.erowid.org/archive/rhodium/chemistry/chloroacetic.html</ref>. |
: CH<sub>3</sub>COOH + Cl<sub>2</sub> → ClCH<sub>2</sub>COOH + HCl | : CH<sub>3</sub>COOH + Cl<sub>2</sub> → ClCH<sub>2</sub>COOH + HCl | ||
| Line 140: | Line 148: | ||
*Make [[glycolic acid]] | *Make [[glycolic acid]] | ||
*Make [[thioglycolic acid]] | *Make [[thioglycolic acid]] | ||
| + | *Make [[nitromethane]] | ||
==Handling== | ==Handling== | ||
===Safety=== | ===Safety=== | ||
| − | Chloroacetic acid is toxic by inhalation, ingestion and skin contact. It is corrosive to metals and tissue. Wear gloves when handling the compound, and make sure you do not breathe the fumes. | + | Chloroacetic acid is toxic by inhalation, ingestion and even skin contact. It is corrosive to metals and tissue. Wear gloves when handling the compound, and make sure you do not breathe the fumes, by wearing a mask or working in a fumehood. |
Chloroacetic acid easily penetrates skin and mucous membranes and interferes with cellular energy production. Initial dermal exposure to high concentrations (e.g., 80% solution) may not appear very damaging at first, however systemic poisoning may present within hours. Exposure can be fatal if greater than 6% body surface area is exposed to chloroacetic acid. The sodium salt does not penetrate the skin as well as the acid but can be as damaging given a longer duration and greater surface area of exposure. | Chloroacetic acid easily penetrates skin and mucous membranes and interferes with cellular energy production. Initial dermal exposure to high concentrations (e.g., 80% solution) may not appear very damaging at first, however systemic poisoning may present within hours. Exposure can be fatal if greater than 6% body surface area is exposed to chloroacetic acid. The sodium salt does not penetrate the skin as well as the acid but can be as damaging given a longer duration and greater surface area of exposure. | ||
Latest revision as of 19:47, 15 September 2022
| |
| Names | |
|---|---|
| IUPAC name
Chloroethanoic acid
| |
| Preferred IUPAC name
Chloroethanoic acid | |
| Systematic IUPAC name
Chloroethanoic acid | |
| Other names
2-Chloroacetic acid
α-Chloroacetic acid | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C2H3ClO2 ClCH2COOH | |
| Molar mass | 94.49 g/mol |
| Appearance | Colorless or white crystals |
| Odor | Vinegar-like |
| Density | 1.58 g/cm3 |
| Melting point | 63 °C (145 °F; 336 K) |
| Boiling point | 189.3 °C (372.7 °F; 462.4 K) |
| 85.8 g/100ml (25 °C) | |
| Solubility | Reacts with alkalis, amines Soluble in acetone, benzene, chloroform, diethyl ether, ethanol, methanol, tetrahydrofuran, toluene Slightly soluble in carbon tetrachloride, hexane |
| Vapor pressure | 6.50·10-2 mmHg at 25 °C |
| Acidity (pKa) | 2.86 |
| Thermochemistry | |
| Std molar
entropy (S |
325.9 J·K-1·mol-1 |
| Std enthalpy of
formation (ΔfH |
-490.1 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 126 °C (259 °F; 399 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
165 mg/kg (mouse, oral) |
| Related compounds | |
| Related compounds
|
Acetic acid Fluoroacetic acid Bromoacetic acid Iodoacetic acid Dichloroacetic acid Trichloroacetic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chloroacetic acid or monochloroacetic acid (MCA), is an organochlorine compound with the formula ClCH2COOH.
Contents
Properties
Chemical
Hydrolysis of chloroacetic acid will yield glycolic acid.
- ClCH2COOH + 2 NaOH → OH-CH2COONa + NaCl + H2O
If the temperature is kept low, the hydrolysis of chloroacetic acid is limited. Since acid-base neutralization is exothermic, it's difficult to maintain a constant temperature. As such, glycolate-free chloroacetate salts can only be made safely by reacting the titular acid with metal carbonates or bicarbonates.
Reaction of sodium chloroacetate with sodium nitrite in aqueous solution will yield nitromethane:[1]
- ClCH2COONa + NaNO2 + H2O → CH3NO2 + NaCl + NaHCO3
Chloroacetic acid will react with sodium thiosulfate to form a Bunte salt, which yields thioglycolic acid
Physical
Chloroacetic acid is a colorless solid, very soluble in water.
Availability
Chloroacetic acid is difficult to acquire in some countries, as it's very toxic. Its salts however, are much more accessible and safer to handle and can be bought online.
Preparation
Chloroacetic acid is prepared industrially via two routes.
The predominant method involves chlorination of glacial acetic acid, with acetic anhydride as a catalyst, in the presence of UV light. Sulfur can also be used as catalyst[2], as can red phosphorus[3].
- CH3COOH + Cl2 → ClCH2COOH + HCl
This reaction also produces acetyl chloride as side product, which may be recovered.
The other main industrial route to chloroacetic acid is hydrolysis of trichloroethylene using sulfuric acid as a catalyst.
- CCl2=CHCl + 2 H2O → ClCH2COOH + 2 HCl
The hydrolysis method produces a highly pure product, which can be important since mono-, di-, and trichloroacetic acids are difficult to separate by distillation.
Projects
- Make glycolic acid
- Make thioglycolic acid
- Make nitromethane
Handling
Safety
Chloroacetic acid is toxic by inhalation, ingestion and even skin contact. It is corrosive to metals and tissue. Wear gloves when handling the compound, and make sure you do not breathe the fumes, by wearing a mask or working in a fumehood.
Chloroacetic acid easily penetrates skin and mucous membranes and interferes with cellular energy production. Initial dermal exposure to high concentrations (e.g., 80% solution) may not appear very damaging at first, however systemic poisoning may present within hours. Exposure can be fatal if greater than 6% body surface area is exposed to chloroacetic acid. The sodium salt does not penetrate the skin as well as the acid but can be as damaging given a longer duration and greater surface area of exposure.
The antidote of chloroacetic acid poisoning is sodium dichloroacetate (50 mg/kg IV over 10 mins, repeated in 2h; double dosage if hemodialysis is performed).
Storage
Chloroacetic acid should be kept in closed bottles with a proper label.
Disposal
Chlorocetic acid can be neutralized with an excess of sodium hydroxide to sodium glycolate, which can be poured down the drain.
References
- ↑ http://www.orgsyn.org/demo.aspx?prep=CV1P0401
- ↑ http://www.prepchem.com/synthesis-of-chloroacetic-acid/
- ↑ https://www.erowid.org/archive/rhodium/chemistry/chloroacetic.html
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Organochlorine compounds
- Acids
- Mid-strength acids
- Carboxylic acids
- Contact poisons