Difference between revisions of "Borax"
m (→Projects) |
|||
| (9 intermediate revisions by 2 users not shown) | |||
| Line 7: | Line 7: | ||
| OtherNames = Disodium tetraborate<br>Sodium tetraborate | | OtherNames = Disodium tetraborate<br>Sodium tetraborate | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = BORAX.jpg |
| ImageSize = | | ImageSize = | ||
| ImageAlt = | | ImageAlt = | ||
| Line 47: | Line 47: | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| AtmosphericOHRateConstant = | | AtmosphericOHRateConstant = | ||
| − | | Appearance = | + | | Appearance = White crystalline solid |
| BoilingPt = | | BoilingPt = | ||
| BoilingPtC = 1,575 | | BoilingPtC = 1,575 | ||
| BoilingPt_ref = | | BoilingPt_ref = | ||
| BoilingPt_notes = | | BoilingPt_notes = | ||
| − | | Density = | + | | Density = 1.73 g/cm<sup>3</sup> |
| Formula = Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub> (anhydrous)<br>Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub>·10H<sub>2</sub>O (decahydrate) | | Formula = Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub> (anhydrous)<br>Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub>·10H<sub>2</sub>O (decahydrate) | ||
| HenryConstant = | | HenryConstant = | ||
| Line 60: | Line 60: | ||
| MeltingPtC = 743 | | MeltingPtC = 743 | ||
| MeltingPt_ref = | | MeltingPt_ref = | ||
| − | | MeltingPt_notes = ( | + | | MeltingPt_notes = (anhydrous) |
| + | | Odor = Odorless | ||
| pKa = | | pKa = | ||
| pKb = | | pKb = | ||
| − | | Solubility = | + | | Solubility = 2.7 g/100 ml (20 °C)<ref>http://www.paulslab.com/img/che/borax-graph-1-720.jpg</ref> |
| − | | SolubleOther = | + | | SolubleOther = Soluble in [[ethylene glycol]], [[glycerol]]<br>Moderate soluble in [[diethylene glycol]], [[methanol]]<br>Slightly soluble in [[acetone]], [[ethanol]], [[ethyl acetate]] |
| Solvent = | | Solvent = | ||
| − | | VaporPressure = | + | | VaporPressure = ~0 mmHg |
}} | }} | ||
| Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| Line 87: | Line 88: | ||
}} | }} | ||
| Section6 = {{Chembox Hazards | | Section6 = {{Chembox Hazards | ||
| − | | AutoignitionPt = | + | | AutoignitionPt = Non-flammable |
| − | | ExploLimits = | + | | ExploLimits = Non-explosive |
| − | | ExternalMSDS = | + | | ExternalMSDS = [https://www.docdroid.net/a74b9FG/borax-sa.pdf.html Sigma-Aldrich] |
| − | | FlashPt = | + | | FlashPt = Non-flammable |
| LD50 = | | LD50 = | ||
| LC50 = | | LC50 = | ||
| Line 104: | Line 105: | ||
| OtherFunction = | | OtherFunction = | ||
| OtherFunction_label = | | OtherFunction_label = | ||
| − | | OtherCompounds = | + | | OtherCompounds = [[Boric acid]] |
}} | }} | ||
}} | }} | ||
| Line 112: | Line 113: | ||
===Chemical=== | ===Chemical=== | ||
Sodium borate will react with a strong acid to release [[boric acid]]. | Sodium borate will react with a strong acid to release [[boric acid]]. | ||
| + | |||
| + | : Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub>·10H<sub>2</sub>O + 2 HCl → 4 H<sub>3</sub>BO<sub>3</sub> + 2 NaCl + 5 H<sub>2</sub>O | ||
| + | |||
| + | Addition of [[hydrogen peroxide]], [[sodium hydroxide]] to sodium borate pentahydrate will give [[sodium perborate]]. | ||
| + | |||
| + | : Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub>·5 H<sub>2</sub>O + NaOH + 2 H<sub>2</sub>O<sub>2</sub> → Na<sub>2</sub>B<sub>2</sub>O<sub>4</sub>(OH)<sub>4</sub> + NaOH + 5 H<sub>2</sub>O + 3/2 O<sub>2</sub> | ||
| + | |||
| + | When borax is added to a flame, it gives a yellow-green color. Since the yellow flame of sodium is undesired, boric acid is used in fireworks instead of borax for green flame and as solution in methanol for pure green flames. | ||
| + | |||
| + | Borax is a prime material in the manufacturing of borosilicate glass. Unlike boric acid, it is not volatile and can be used in high temperature chemical reactions as a source of boron. | ||
===Physical=== | ===Physical=== | ||
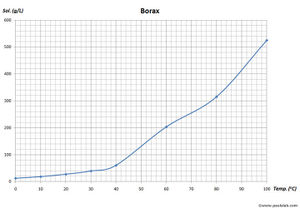
| − | Borax is a white crystalline solid. It melts at 743 °C and boils at 1,575 °C. It has a density of 1.73 g/cm<sup>3</sup>. | + | Borax is a white crystalline solid. It is odorless, and has an unpleasant taste. Borax melts at 743 °C and boils at 1,575 °C. It has a density of 1.73 g/cm<sup>3</sup>. Borax is poorly soluble in cold water, but it's solubility increases with temperature. It is soluble in some organic solvents, like [[ethylene glycol]], [[glycerol]], while moderate soluble in [[diethylene glycol]], [[methanol]]. Borax is only slightly soluble in more common solvents like [[acetone]], [[ethanol]], [[ethyl acetate]]. |
| + | [[File:Borax water solubility graphic.jpg|300px|thumb|Water solubility of borax]] | ||
==Availability== | ==Availability== | ||
| − | Borax is sold by various chemical suppliers. | + | Borax is sold by various chemical suppliers. Can also be acquired online. |
| − | It is also sold in various pharmacies and | + | It is also sold in various pharmacies and iron forgeries. |
==Preparation== | ==Preparation== | ||
| − | Sodium borate can be made by reacting boric acid with | + | Sodium borate can be made by reacting boric acid with [[sodium hydroxide]], under continuous stirring. |
| + | |||
| + | :4 H<sub>3</sub>BO<sub>3</sub> + 2 NaOH → Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub> + 7 H<sub>2</sub>O | ||
==Projects== | ==Projects== | ||
*Make [[boric acid]] | *Make [[boric acid]] | ||
| − | *Grow borax crystals,borax snowflakes | + | *Grow borax crystals, borax snowflakes |
| + | *pH buffer | ||
| + | *Water-softening agent | ||
| + | *Flux for melting metals | ||
| + | *Borax method (gold extraction) | ||
| + | *Fire retardant | ||
| + | *Make [[sodium perborate]] | ||
| + | *Curing agent (leather) | ||
==Handling== | ==Handling== | ||
===Safety=== | ===Safety=== | ||
| − | Borax has low toxicity. | + | Borax has low toxicity. It is only mildly irritant to skin. |
Borax was added to the Substance of Very High Concern (SVHC) candidate list on 16 December 2010 in the EU. | Borax was added to the Substance of Very High Concern (SVHC) candidate list on 16 December 2010 in the EU. | ||
| Line 153: | Line 174: | ||
[[Category:Minerals]] | [[Category:Minerals]] | ||
[[Category:Readily available chemicals]] | [[Category:Readily available chemicals]] | ||
| + | [[Category:Chemicals for crystal growing]] | ||
| + | [[Category:Irritants]] | ||
Latest revision as of 15:32, 9 May 2022

| |
| Names | |
|---|---|
| IUPAC name
Sodium tetraborate decahydrate
| |
| Systematic IUPAC name
Sodium borate | |
| Other names
Disodium tetraborate
Sodium tetraborate | |
| Properties | |
| Na2B4O7 (anhydrous) Na2B4O7·10H2O (decahydrate) | |
| Molar mass | 381.38 g/mol (decahydrate) 201.22 g/mol (anhydrate) |
| Appearance | White crystalline solid |
| Odor | Odorless |
| Density | 1.73 g/cm3 |
| Melting point | 743 °C (1,369 °F; 1,016 K) (anhydrous) |
| Boiling point | 1,575 °C (2,867 °F; 1,848 K) |
| 2.7 g/100 ml (20 °C)[1] | |
| Solubility | Soluble in ethylene glycol, glycerol Moderate soluble in diethylene glycol, methanol Slightly soluble in acetone, ethanol, ethyl acetate |
| Vapor pressure | ~0 mmHg |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | Non-flammable |
| Related compounds | |
| Related compounds
|
Boric acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Borax or sodium borate, sodium tetraborate, is an important boron compound. It has the chemical formula Na2B4O7.
Contents
Properties
Chemical
Sodium borate will react with a strong acid to release boric acid.
- Na2B4O7·10H2O + 2 HCl → 4 H3BO3 + 2 NaCl + 5 H2O
Addition of hydrogen peroxide, sodium hydroxide to sodium borate pentahydrate will give sodium perborate.
- Na2B4O7·5 H2O + NaOH + 2 H2O2 → Na2B2O4(OH)4 + NaOH + 5 H2O + 3/2 O2
When borax is added to a flame, it gives a yellow-green color. Since the yellow flame of sodium is undesired, boric acid is used in fireworks instead of borax for green flame and as solution in methanol for pure green flames.
Borax is a prime material in the manufacturing of borosilicate glass. Unlike boric acid, it is not volatile and can be used in high temperature chemical reactions as a source of boron.
Physical
Borax is a white crystalline solid. It is odorless, and has an unpleasant taste. Borax melts at 743 °C and boils at 1,575 °C. It has a density of 1.73 g/cm3. Borax is poorly soluble in cold water, but it's solubility increases with temperature. It is soluble in some organic solvents, like ethylene glycol, glycerol, while moderate soluble in diethylene glycol, methanol. Borax is only slightly soluble in more common solvents like acetone, ethanol, ethyl acetate.
Availability
Borax is sold by various chemical suppliers. Can also be acquired online.
It is also sold in various pharmacies and iron forgeries.
Preparation
Sodium borate can be made by reacting boric acid with sodium hydroxide, under continuous stirring.
- 4 H3BO3 + 2 NaOH → Na2B4O7 + 7 H2O
Projects
- Make boric acid
- Grow borax crystals, borax snowflakes
- pH buffer
- Water-softening agent
- Flux for melting metals
- Borax method (gold extraction)
- Fire retardant
- Make sodium perborate
- Curing agent (leather)
Handling
Safety
Borax has low toxicity. It is only mildly irritant to skin.
Borax was added to the Substance of Very High Concern (SVHC) candidate list on 16 December 2010 in the EU.
Storage
Borax should be stored in closed bottles
Disposal
Sodium tetraborate can be dumped in trash.