Difference between revisions of "Guanidine"
Diachrynic (Talk | contribs) m |
|||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 64: | Line 64: | ||
| pKa = 12.5 | | pKa = 12.5 | ||
| pKb = | | pKb = | ||
| − | | Solubility = | + | | Solubility = |
| SolubleOther = Soluble in polar solvents | | SolubleOther = Soluble in polar solvents | ||
| Solvent = | | Solvent = | ||
| Line 105: | Line 105: | ||
| OtherFunction = | | OtherFunction = | ||
| OtherFunction_label = | | OtherFunction_label = | ||
| − | | OtherCompounds = [[Ammonia]]<br>[[Hydrazine]]<br>[[Urea]] | + | | OtherCompounds = [[Ammonia]]<br>[[Hydrazine]]<br>[[Methylamine]]<br>[[Urea]] |
}} | }} | ||
}} | }} | ||
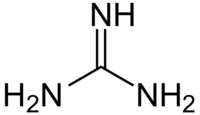
'''Guanidine''' is an organic compound, a base with the formula '''HNC(NH<sub>2</sub>)<sub>2</sub>'''. Guanidine is sometimes shortened to '''Gdn'''. | '''Guanidine''' is an organic compound, a base with the formula '''HNC(NH<sub>2</sub>)<sub>2</sub>'''. Guanidine is sometimes shortened to '''Gdn'''. | ||
| + | |||
| + | Guanidine salts are often written as "guanidine + name of the anion" (guanidine nitrate e.g.), which is incorrect, as the terminology for protonated amines demands the suffix "-ium" be used (you won't say "ammonia nitrate", now, won't you?). However, due to the fact that this (incorrect) terminology is very often encountered the case of other organic base salts, it's usually tolerated. | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| + | Guanidine is a strong monobasic Brønsted base, which readily absorbs [[water]] and [[carbon dioxide]] from air. It can form strongly alkaline solutions with water and alcohols. Aqueous solutions with a concentration of 20% have a pH of 13.5 at 25 °C. | ||
| + | |||
| + | If an aqueous solution of guanidine is heated, guanidine will hydrolyze to [[urea]].<ref>https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a12_545.pub2</ref>. | ||
| + | |||
As a base, guanidine will react with acids to form salts. By protanating guanidine, the [[guanidinium]] ion is formed. | As a base, guanidine will react with acids to form salts. By protanating guanidine, the [[guanidinium]] ion is formed. | ||
| − | :HNC(NH<sub>2</sub>)<sub>2</sub> + HCl → C(NH<sub>2</sub>)<sub>3</sub><sup>+</sup>Cl<sup>-</sup> | + | : HNC(NH<sub>2</sub>)<sub>2</sub> + HCl → C(NH<sub>2</sub>)<sub>3</sub><sup>+</sup>Cl<sup>-</sup> |
| + | |||
| + | Solutions of guanidine in water or other solvents are stable if kept airtight. | ||
===Physical=== | ===Physical=== | ||
| − | Guanidine is a solid white hygroscopic compound, | + | Guanidine is a solid white hygroscopic/extremely deliquescent compound,<ref>Marckwald, W. and Struwe, F. (1922), Über einige Guanidoniumsalze. Ber. dtsch. Chem. Ges. A/B, 55: 457-463. [https://doi.org/10.1002/cber.19220550221 doi:10.1002/cber.19220550221]</ref> highly soluble in water and soluble in organic solvents. |
==Availability== | ==Availability== | ||
| Line 129: | Line 137: | ||
To obtain water-free freebase guanidine, a simple method is to add a guanidinium salt, like guanidinium chloride or sulfate to a methanolic solution of [[sodium methoxide]] (which can be easily made by adding [[sodium]] metal to dried [[methanol]]). The sodium salt will precipitate out of the solution, while the freebase guanidine will remain in solution. | To obtain water-free freebase guanidine, a simple method is to add a guanidinium salt, like guanidinium chloride or sulfate to a methanolic solution of [[sodium methoxide]] (which can be easily made by adding [[sodium]] metal to dried [[methanol]]). The sodium salt will precipitate out of the solution, while the freebase guanidine will remain in solution. | ||
| − | :C(NH<sub>2</sub>)<sub>3</sub>Cl + CH<sub>3</sub>ONa → HNC(NH<sub>2</sub>)<sub>2</sub> + CH<sub>3</sub>OH + NaCl | + | : C(NH<sub>2</sub>)<sub>3</sub>Cl + CH<sub>3</sub>ONa → HNC(NH<sub>2</sub>)<sub>2</sub> + CH<sub>3</sub>OH + NaCl |
Heat the MeOH solution to drive off excess solvent, then cool it to obtain the crystallized pure compound. | Heat the MeOH solution to drive off excess solvent, then cool it to obtain the crystallized pure compound. | ||
| Line 135: | Line 143: | ||
For the preparation of guanidinium salts, check the page for each compound. | For the preparation of guanidinium salts, check the page for each compound. | ||
| − | Guanidine can also be obtained from oxidative degradation of guanine, isolated from Peruvian guano (hence its name). Guano fertilizer can be bought from many hardware and gardening stores. | + | Guanidine can also be obtained from oxidative degradation of guanine, isolated from Peruvian guano (hence its name). Guano fertilizer can be bought from many hardware and gardening stores. Yields from this route however, are not that great. |
| + | |||
| + | [[Arginine]] has a guanidine group, which can be removed via dissociation. | ||
==Projects== | ==Projects== | ||
*Make rocket fuel | *Make rocket fuel | ||
*Make guanidinium salts ([[guanidinium carbonate]], [[guanidinium chloride]], [[guanidinium nitrate]], [[guanidinium perchlorate]], [[guanidinium sulfate]]) | *Make guanidinium salts ([[guanidinium carbonate]], [[guanidinium chloride]], [[guanidinium nitrate]], [[guanidinium perchlorate]], [[guanidinium sulfate]]) | ||
| + | *Make various organic compounds | ||
==Handling== | ==Handling== | ||
| Line 146: | Line 157: | ||
===Storage=== | ===Storage=== | ||
| − | Guanidine free base should be kept in closed bottles, in a dry place. | + | Guanidine free base should be kept in airtight closed bottles, in a dry place. |
===Disposal=== | ===Disposal=== | ||
Latest revision as of 19:02, 27 March 2022
| |
| Names | |
|---|---|
| IUPAC names
Guanidine
Iminomethanediamine | |
| Other names
Aminomethanamidine
Carbamamidine Carbamidine Gdn Guanidin Imidourea | |
| Properties | |
| CH5N3 HNC(NH2)2 | |
| Molar mass | 59.07 g/mol |
| Appearance | Hygroscopic colorless solid |
| Odor | Odorless |
| Density | 1.6 g/cm3 |
| Melting point | 50 °C (122 °F; 323 K) |
| Boiling point | Decomposes |
| Solubility | Soluble in polar solvents |
| Vapor pressure | 2.2 mmHg at 25 °C |
| Acidity (pKa) | 12.5 |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
−57 – −55 kJ/mol |
| Hazards | |
| Safety data sheet | Fluorochem |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
475 mg/kg (rat, oral)[1] |
| Related compounds | |
| Related compounds
|
Ammonia Hydrazine Methylamine Urea |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Guanidine is an organic compound, a base with the formula HNC(NH2)2. Guanidine is sometimes shortened to Gdn.
Guanidine salts are often written as "guanidine + name of the anion" (guanidine nitrate e.g.), which is incorrect, as the terminology for protonated amines demands the suffix "-ium" be used (you won't say "ammonia nitrate", now, won't you?). However, due to the fact that this (incorrect) terminology is very often encountered the case of other organic base salts, it's usually tolerated.
Contents
Properties
Chemical
Guanidine is a strong monobasic Brønsted base, which readily absorbs water and carbon dioxide from air. It can form strongly alkaline solutions with water and alcohols. Aqueous solutions with a concentration of 20% have a pH of 13.5 at 25 °C.
If an aqueous solution of guanidine is heated, guanidine will hydrolyze to urea.[2].
As a base, guanidine will react with acids to form salts. By protanating guanidine, the guanidinium ion is formed.
- HNC(NH2)2 + HCl → C(NH2)3+Cl-
Solutions of guanidine in water or other solvents are stable if kept airtight.
Physical
Guanidine is a solid white hygroscopic/extremely deliquescent compound,[3] highly soluble in water and soluble in organic solvents.
Availability
Unlike its salts, free base guanidine is difficult to find. Fluorochem and Oakwood Chemical are one of the few suppliers that sell the free base compound.
Guanidinium salts, of the other hand, can be purchased from many chemical suppliers. To obtain the free base, simply react the salt with a stronger base then extract the resulting guanidine.
Preparation
Free base guanidine can be prepared from guanidinium salts by adding sodium hydroxide to said guanidinium salt, then recrystallizing it from the solvent.
To obtain water-free freebase guanidine, a simple method is to add a guanidinium salt, like guanidinium chloride or sulfate to a methanolic solution of sodium methoxide (which can be easily made by adding sodium metal to dried methanol). The sodium salt will precipitate out of the solution, while the freebase guanidine will remain in solution.
- C(NH2)3Cl + CH3ONa → HNC(NH2)2 + CH3OH + NaCl
Heat the MeOH solution to drive off excess solvent, then cool it to obtain the crystallized pure compound.
For the preparation of guanidinium salts, check the page for each compound.
Guanidine can also be obtained from oxidative degradation of guanine, isolated from Peruvian guano (hence its name). Guano fertilizer can be bought from many hardware and gardening stores. Yields from this route however, are not that great.
Arginine has a guanidine group, which can be removed via dissociation.
Projects
- Make rocket fuel
- Make guanidinium salts (guanidinium carbonate, guanidinium chloride, guanidinium nitrate, guanidinium perchlorate, guanidinium sulfate)
- Make various organic compounds
Handling
Safety
Guanidine and its salts aren't volatile or very toxic and don't require special handling. Freebase guanidine may be irritant, so make sure to wear gloves when handling it. Unfortunately, there isn't much information in literature about its toxicity or prolonged exposure.
Storage
Guanidine free base should be kept in airtight closed bottles, in a dry place.
Disposal
No special disposal is required. Some guanidinium salts are even used as fertilizer.
References
- ↑ https://www.drugbank.ca/drugs/DB00536
- ↑ https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a12_545.pub2
- ↑ Marckwald, W. and Struwe, F. (1922), Über einige Guanidoniumsalze. Ber. dtsch. Chem. Ges. A/B, 55: 457-463. doi:10.1002/cber.19220550221