Difference between revisions of "Propionic acid"
| (4 intermediate revisions by 2 users not shown) | |||
| Line 12: | Line 12: | ||
| ImageName = | | ImageName = | ||
| ImageCaption = Propionic acid obtained by NurdRage from the haloform reaction of MEK and NaOCl | | ImageCaption = Propionic acid obtained by NurdRage from the haloform reaction of MEK and NaOCl | ||
| − | | ImageFile1 = | + | | ImageFile1 = Propionic acid structural formula.png |
| − | | ImageSize1 = | + | | ImageSize1 = 100 |
| ImageAlt1 = | | ImageAlt1 = | ||
| ImageName1 = | | ImageName1 = | ||
| + | | ImageCaption1 = Structure | ||
| ImageFile2 = | | ImageFile2 = | ||
| ImageSize2 = | | ImageSize2 = | ||
| Line 44: | Line 45: | ||
| 3DMet = | | 3DMet = | ||
| Abbreviations = | | Abbreviations = | ||
| − | | SMILES = | + | | SMILES = CCC(=O)O |
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| Line 56: | Line 57: | ||
| Formula = C<sub>3</sub>H<sub>6</sub>O<sub>2</sub> | | Formula = C<sub>3</sub>H<sub>6</sub>O<sub>2</sub> | ||
| HenryConstant = 4.45·10<sup>−4</sup> L·atm/mol | | HenryConstant = 4.45·10<sup>−4</sup> L·atm/mol | ||
| − | | LogP = | + | | LogP = 0.33 |
| MolarMass = 74.08 g/mol | | MolarMass = 74.08 g/mol | ||
| MeltingPt = | | MeltingPt = | ||
| Line 66: | Line 67: | ||
| pKb = | | pKb = | ||
| Solubility = 8.19 g/g (−28.3 °C)<br>34.97 g/g (−23.9 °C)<br>Miscible (> −19.3 °C) | | Solubility = 8.19 g/g (−28.3 °C)<br>34.97 g/g (−23.9 °C)<br>Miscible (> −19.3 °C) | ||
| − | | SolubleOther = Miscible with [[chloroform]], [[dichloromethane]], [[diethyl ether]], [[ethanol]] | + | | SolubleOther = Reacts with alkali, amines<br>Miscible with glacial [[acetic acid]], [[acetone]], [[benzene]], [[chloroform]], [[dichloromethane]], [[diethyl ether]], [[Dimethyl sulfoxide|DMSO]], [[ethanol]] |
| Solvent = | | Solvent = | ||
| VaporPressure = 0.32 kPa (20 °C)<br>0.47 kPa (25 °C)<br>9.62 kPa (100 °C) | | VaporPressure = 0.32 kPa (20 °C)<br>0.47 kPa (25 °C)<br>9.62 kPa (100 °C) | ||
| Line 132: | Line 133: | ||
None of the above however are cheap enough to be done at home. | None of the above however are cheap enough to be done at home. | ||
| − | A more accessible route involves the addition of a strong acid to calcium propionate. Conc. [[sulfuric acid]] may not be a good choice, as the resulting [[calcium sulfate]] will tend to trap the propionic acid in it's particulates. Calcium propionate is | + | A more accessible route involves the addition of a strong acid to [[calcium propionate]]. Conc. [[sulfuric acid]] may not be a good choice, as the resulting [[calcium sulfate]] will tend to trap the propionic acid in it's particulates, and it's difficult to filter. Instead, [[hydrochloric acid]] can be used, then the resulting propionic acid-water azeotrope is distilled, the propionic acid is converted again to salt, the solution is dried to recovered the solid propionate salt, which is further dried, then the pure propionic acid is obtained by adding conc. sulfuric acid to the salt and the propionic acid is distilled. Calcium propionate is available in many places as food preservative (E 282) and can be cheaply bought in large quantities, thus making this method attractive. |
Propionic acid is a side product from the [[haloform reaction]] of [[methyl ethyl ketone]] with bleach. Since bleach is diluted, you will end up with a very diluted solution of sodium propionate. Depending on how much liquid you want to distill, you can either concentrate the solution by heating it at 90 °C for many hours until the volume of liquid is low enough, add HCl then distill, or you can simply add the acid to the diluted solution and fractionally distill the propionic acid, like NurdRage did<ref>https://www.youtube.com/watch?v=iDMuQtglPIw</ref>. Propanoic acid forms an azeotrope with water consisting of 17% CH<sub>3</sub>CH<sub>2</sub>COOH with the rest being water which boils at 99.96 °C, meaning the distillate will be very diluted propanoic acid. Neutralize the diluted propionic acid with baking soda to form sodium propionate again. Remove the water by boiling away the water and dry the propanoate. Once dried, add conc. [[sulfuric acid]] (98%) and fractionally distill the resulting acid, as impurities may still be in the crude propionate salt. Dry the crude propionic acid with anh. [[sodium sulfate]] and distill it again. | Propionic acid is a side product from the [[haloform reaction]] of [[methyl ethyl ketone]] with bleach. Since bleach is diluted, you will end up with a very diluted solution of sodium propionate. Depending on how much liquid you want to distill, you can either concentrate the solution by heating it at 90 °C for many hours until the volume of liquid is low enough, add HCl then distill, or you can simply add the acid to the diluted solution and fractionally distill the propionic acid, like NurdRage did<ref>https://www.youtube.com/watch?v=iDMuQtglPIw</ref>. Propanoic acid forms an azeotrope with water consisting of 17% CH<sub>3</sub>CH<sub>2</sub>COOH with the rest being water which boils at 99.96 °C, meaning the distillate will be very diluted propanoic acid. Neutralize the diluted propionic acid with baking soda to form sodium propionate again. Remove the water by boiling away the water and dry the propanoate. Once dried, add conc. [[sulfuric acid]] (98%) and fractionally distill the resulting acid, as impurities may still be in the crude propionate salt. Dry the crude propionic acid with anh. [[sodium sulfate]] and distill it again. | ||
| Line 140: | Line 141: | ||
Another method involves the reaction of an ethyl magnesium halide with [[carbon dioxide]]. This produces propionic acid which can be recovered from the reaction mass in a similar way to the method above (except less water). | Another method involves the reaction of an ethyl magnesium halide with [[carbon dioxide]]. This produces propionic acid which can be recovered from the reaction mass in a similar way to the method above (except less water). | ||
| − | Propionibacterium is known to produce propionic acid (it gives sweat its bad smell), though it produces too little to be a | + | Propionibacterium is known to produce propionic acid (it gives sweat its bad smell), though it produces too little to be a viable source. |
==Projects== | ==Projects== | ||
*Make ethyl propionate | *Make ethyl propionate | ||
*Make calcium propionate | *Make calcium propionate | ||
| + | *Make cellulose-acetate-propionate thermoplastic | ||
==Handling== | ==Handling== | ||
| Line 176: | Line 178: | ||
[[Category:Volatile chemicals]] | [[Category:Volatile chemicals]] | ||
[[Category:Liquids]] | [[Category:Liquids]] | ||
| + | [[Category:Irritants]] | ||
Latest revision as of 19:14, 12 September 2021
 Propionic acid obtained by NurdRage from the haloform reaction of MEK and NaOCl
| |
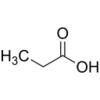 Structure
| |
| Names | |
|---|---|
| IUPAC name
Propanoic acid
| |
| Other names
Carboxyethane
Ethanecarboxylic acid Ethylformic acid Methylacetic acid | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C3H6O2 | |
| Molar mass | 74.08 g/mol |
| Appearance | Colorless liquid |
| Odor | Unpleasant, rancid, body-odor-like |
| Density | 0.98797 g/cm3 |
| Melting point | −20.5 °C (−4.9 °F; 252.7 K) |
| Boiling point | 141.15 °C (286.07 °F; 414.30 K) |
| 8.19 g/g (−28.3 °C) 34.97 g/g (−23.9 °C) Miscible (> −19.3 °C) | |
| Solubility | Reacts with alkali, amines Miscible with glacial acetic acid, acetone, benzene, chloroform, dichloromethane, diethyl ether, DMSO, ethanol |
| Vapor pressure | 0.32 kPa (20 °C) 0.47 kPa (25 °C) 9.62 kPa (100 °C) |
| Acidity (pKa) | 4.88 |
| Thermochemistry | |
| Std molar
entropy (S |
191 J·mol-1·K-1 |
| Std enthalpy of
formation (ΔfH |
−510.8 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 54 °C |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
1,370 mg/kg (mouse, oral) |
| Related compounds | |
| Related compounds
|
Acetic acid Butyric acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Propionic acid or propanoic acid is a naturally occurring carboxylic acid with chemical formula CH3CH2COOH. It is a clear liquid with a pungent and unpleasant smell, somewhat resembling body odor.
Contents
Properties
Chemical
Propionic acid reacts with bases to form salts known as propionates. Reaction with calcium hydroxide gives calcium propionate.
- 2 CH3CH2COOH + Ca(OH)2 → Ca(CH3CH2COO)2 + 2 H2O
Physical
Propionic acid is a colorless liquid, miscible with water and organic solvents. It has a rancid smell, reminiscent of body sweat. It can be salted out from solution by adding salt.
Availability
Propionic acid is sold by chemical suppliers. It can also be bought from eBay.
Preparation
Propionic acid is industrially produced by several methods.
One route involves hydrocarboxylation of ethylene using nickel carbonyl as catalyst.
Another involves aerobic oxidation of propionaldehyde, at temperatures between 40–50 °C.
None of the above however are cheap enough to be done at home.
A more accessible route involves the addition of a strong acid to calcium propionate. Conc. sulfuric acid may not be a good choice, as the resulting calcium sulfate will tend to trap the propionic acid in it's particulates, and it's difficult to filter. Instead, hydrochloric acid can be used, then the resulting propionic acid-water azeotrope is distilled, the propionic acid is converted again to salt, the solution is dried to recovered the solid propionate salt, which is further dried, then the pure propionic acid is obtained by adding conc. sulfuric acid to the salt and the propionic acid is distilled. Calcium propionate is available in many places as food preservative (E 282) and can be cheaply bought in large quantities, thus making this method attractive.
Propionic acid is a side product from the haloform reaction of methyl ethyl ketone with bleach. Since bleach is diluted, you will end up with a very diluted solution of sodium propionate. Depending on how much liquid you want to distill, you can either concentrate the solution by heating it at 90 °C for many hours until the volume of liquid is low enough, add HCl then distill, or you can simply add the acid to the diluted solution and fractionally distill the propionic acid, like NurdRage did[1]. Propanoic acid forms an azeotrope with water consisting of 17% CH3CH2COOH with the rest being water which boils at 99.96 °C, meaning the distillate will be very diluted propanoic acid. Neutralize the diluted propionic acid with baking soda to form sodium propionate again. Remove the water by boiling away the water and dry the propanoate. Once dried, add conc. sulfuric acid (98%) and fractionally distill the resulting acid, as impurities may still be in the crude propionate salt. Dry the crude propionic acid with anh. sodium sulfate and distill it again.
Oxidation of propanol with a strong oxidizer, such as potassium permanganate will give propionic acid.
Another method involves the reaction of an ethyl magnesium halide with carbon dioxide. This produces propionic acid which can be recovered from the reaction mass in a similar way to the method above (except less water).
Propionibacterium is known to produce propionic acid (it gives sweat its bad smell), though it produces too little to be a viable source.
Projects
- Make ethyl propionate
- Make calcium propionate
- Make cellulose-acetate-propionate thermoplastic
Handling
Safety
Concentrated propionic acid is corrosive and should be handled with proper protection. It also has a bad smell and work should be done outside or in a fumehood.
Storage
Propanoic acid should be kept in sealed bottles, to avoid releasing its bad smell in the workplace. A teflon seal capped bottle is good enough.
Disposal
Should be neutralized with a base, such as sodium bicarbonate or calcium hydroxide/carbonate then poured down the drain.
References
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Acids
- Weak acids
- Carboxylic acids
- Solvents
- Polar solvents
- Protic solvents
- Easily prepared chemicals
- Foul smelling compounds
- Volatile chemicals
- Liquids
- Irritants