Difference between revisions of "Trinitrotoluene"
| (3 intermediate revisions by the same user not shown) | |||
| Line 8: | Line 8: | ||
TNT, Trilite, Tolite, Trinol, Trotyl, Tritolo, Tritolol, Triton, Tritone, Trotol, Trinitrotoluol | TNT, Trilite, Tolite, Trinol, Trotyl, Tritolo, Tritolol, Triton, Tritone, Trotol, Trinitrotoluol | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = Trinitrotoluene TNT structure.png |
| − | | ImageSize = | + | | ImageSize = 250 |
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| + | | ImageCaption = TNT structure | ||
| ImageFile1 = | | ImageFile1 = | ||
| ImageSize1 = | | ImageSize1 = | ||
| Line 62: | Line 63: | ||
| MeltingPt_ref = | | MeltingPt_ref = | ||
| MeltingPt_notes = | | MeltingPt_notes = | ||
| + | | Odor = Odorless | ||
| pKa = | | pKa = | ||
| pKb = | | pKb = | ||
| Line 105: | Line 107: | ||
| OtherFunction = | | OtherFunction = | ||
| OtherFunction_label = | | OtherFunction_label = | ||
| − | | OtherCompounds = [[Picric acid]] | + | | OtherCompounds = [[Picric acid]]<br>[[Trinitroaniline]] |
}} | }} | ||
}} | }} | ||
| Line 151: | Line 153: | ||
[[Category:Organic compounds]] | [[Category:Organic compounds]] | ||
[[Category:Aromatic compounds]] | [[Category:Aromatic compounds]] | ||
| − | [[Category: | + | [[Category:Nitro compounds]] |
| + | [[Category:Nitroaromatics]] | ||
[[Category:Materials unstable in basic solution]] | [[Category:Materials unstable in basic solution]] | ||
[[Category:Energetic materials]] | [[Category:Energetic materials]] | ||
[[Category:High explosives]] | [[Category:High explosives]] | ||
[[Category:Secondary explosives]] | [[Category:Secondary explosives]] | ||
Latest revision as of 22:12, 26 October 2020
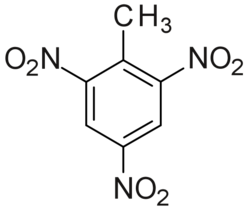 TNT structure
| |
| Names | |
|---|---|
| IUPAC name
2-Methyl-1,3,5-trinitrobenzene
| |
| Other names
2,4,6-Trinitromethylbenzene, 2,4,6-Trinitrotoluene,
TNT, Trilite, Tolite, Trinol, Trotyl, Tritolo, Tritolol, Triton, Tritone, Trotol, Trinitrotoluol
| |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C7H5N3O6 | |
| Molar mass | 227.13 g/mol |
| Appearance | Pale yellow solid |
| Odor | Odorless |
| Density | 1.654 g/cm3 |
| Melting point | 80.35 °C (176.63 °F; 353.50 K) |
| Boiling point | 240 °C (464 °F; 513 K) (decomposes) |
| 0.13 g/L (20 °C) | |
| Solubility | Soluble in diethyl ether, acetone, benzene, pyridine |
| Vapor pressure | 0.0002 mmHg (20°C) |
| Hazards | |
| Safety data sheet | Zaryachem |
| Flash point | 167 °C |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
795 mg/kg (rat, oral) 660 (mouse, oral) |
| Related compounds | |
| Related compounds
|
Picric acid Trinitroaniline |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trinitrotoluene or TNT, or more specifically 2,4,6-trinitrotoluene, is a chemical compound with the formula C6H2(NO2)3CH3. TNT's most common use is that of an explosive material, with both military and (some) civilian applications. The explosive yield of TNT is considered to be the standard measure of strength of explosive materials.
Contents
Properties
Chemical
TNT will burn if ignited, but requires a blasting cap to detonate it.
Physical
TNT is a yellowish solid, insoluble in water but readily soluble in organic solvents.
Explosive
TNT is a relative insensitive explosive, with a RE factor of 1.00 and a detonation velocity of 6900 m/s.
Availability
Chemical suppliers do not sell bulk TNT, nor do they have it in their stock, they will however sell diluted TNT solutions, mainly for analysis. These however are not available to the amateur chemist, nor is it economical to purchase such materials in bulk.
Preparation
TNT can be prepared by nitrating toluene to mononitrotoluene, then dinitrotoluene and finally trinitrotoluene using a nitrating mixture. The synthesis process is fairly complex.
Projects
- Make blasting charge
Handling
Safety
TNT is extremely toxic. Exposure to TNT leads to the skin turning yellow, and people with such condition during WWI (women working in munitions factories) were nicknamed "canaries" or "canary girls".
Storage
TNT should be stored in closed containers, away from any source of hazard.
Disposal
TNT can be mixed with a flammable solvent and burned, or simply burned. Both methods yield lots of carbon monoxide, soot, VOCs, PAHs, other aromatics, etc. This must be done outside. Since the resulting smoke is harmful, it's best to use a special incinerator, equipped with an afterburner.
TNT can be safely neutralized with Fenton's reagent. Slowly adding drops of diluted solutions of TNT in Fenton's solution will limit the aerosolization of TNT and other byproducts from the oxidation. This process is best done in a well ventilated area or (preferably) outside. UV light will accelerate the reaction.[1][2]
References
- ↑ http://nmrlab.yo-que.ch/controversia/lib/exe/fetch.php?media=c2:l2:fenton-_explosivos.pdf
- ↑ https://dl.sciencesocieties.org/publications/jeq/abstracts/26/2/JEQ0260020480?access=0&view=pdf
Sciencemadness library
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Aromatic compounds
- Nitro compounds
- Nitroaromatics
- Materials unstable in basic solution
- Energetic materials
- High explosives
- Secondary explosives