Difference between revisions of "Ethylene glycol dinitrate"
| (4 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
{{stub}} | {{stub}} | ||
| − | '''Ethylene glycol dinitrate''', IUPAC name '''1,2-dinitroxyethane''', also known as '''nitroglycol''' or '''EGDN''', is a pale yellow, syrupy, explosive liquid. In the series of [[alkyl | + | {{Chembox |
| + | | Name = Ethylene glycol dinitrate | ||
| + | | Reference = | ||
| + | | IUPACName = 1,2-dinitroxyethane | ||
| + | | PIN = | ||
| + | | SystematicName = | ||
| + | | OtherNames = 1,2-Bis(nitrooxy)ethane<br>1,2-Ethanediol dinitrate<br>Dinitroglycol<br>EGDN<br>Ethylene dinitrate<br>Ethylene glycol dinitrate<br>Ethylene nitrate<br>Ethane-1,2-diyl dinitrate<br>Glycol dinitrate<br>Nitroglycol | ||
| + | <!-- Images --> | ||
| + | | ImageFile = EGDN structure.png | ||
| + | | ImageSize = 280 | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageFile1 = | ||
| + | | ImageSize1 = | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = Colorless to yellow liquid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 197.5 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = | ||
| + | | Density = 1.4918 g/cm<sup>3</sup> (20 °C) | ||
| + | | Formula = C<sub>2</sub>H<sub>4</sub>N<sub>2</sub>O<sub>6</sub> | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| + | | MolarMass = 152.1 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = −22.3 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | Odor = Odorless | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = 0.5 g/100 ml | ||
| + | | SolubleOther = Miscibile with glacial [[acetic acid]], [[acetone]], [[benzene]], [[carbon tetrachloride|CCl<sub>4</sub>]], [[chloroform]], [[diethyl ether]], [[methanol]], [[toluene]] | ||
| + | | Solvent = | ||
| + | | VaporPressure = 0.05 mmHg (20 °C) | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = 265.9 kcal/mol | ||
| + | | DeltaHf = | ||
| + | | Entropy = | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = Medium | ||
| + | | FrictionSens = Medium | ||
| + | | DetonationV = 8300 m/s | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = | ||
| + | | ExploLimits = | ||
| + | | ExternalMSDS = [http://www.cpcb.nic.in/divisionsofheadoffice/pci-ssi/MATERIAL_SAFETY-DATABASE/MSDS2008/268.pdf cpcb] | ||
| + | | FlashPt = 215 °C (419 °F, 488 K) | ||
| + | | LD50 = | ||
| + | | LC50 = | ||
| + | | MainHazards = Explosive | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Methyl nitrate]]<br>[[Nitroglycerin]] | ||
| + | }} | ||
| + | }} | ||
| + | '''Ethylene glycol dinitrate''', IUPAC name '''1,2-dinitroxyethane''', also known as '''nitroglycol''' or '''EGDN''', is a pale yellow, syrupy, explosive liquid. In the series of [[alkyl nitrate]]s it is among those which have a 1:1 ratio of nitroxy groups per carbon atom, and also exhibits a perfect [[oxygen balance]]. | ||
==Properties== | ==Properties== | ||
| Line 11: | Line 119: | ||
==Preparation== | ==Preparation== | ||
| − | EGDN can be prepared by nitrating ethylene glycol under careful monitoring. | + | EGDN can be prepared by nitrating [[ethylene glycol]] under careful monitoring. |
| + | |||
| + | Another route is the reaction between [[ethylene oxide]] and [[dinitrogen pentoxide]], in the presence of [[ozone]]. Reaction takes place in [[dichloromethane]] for 5 min, at temperatures between 10-15 °C. Yield is 96%.<ref>[http://www.sciencedirect.com/science/article/pii/0040403988852729 Golding, P.; Millar, R. W.; Paul, N. C.; Richards, D. H.; Tetrahedron Letters; vol. 29; nb. 22; (1988); p. 2731 - 2734]</ref><ref>[http://www.sciencedirect.com/science/article/pii/S0040402001879783 Golding, Peter; Millar, Ross W.; Paul, Norman C.; Richards, David H.; Tetrahedron; vol. 49; nb. 32; (1993); p. 7037-7050]</ref> | ||
==Handling== | ==Handling== | ||
| Line 18: | Line 128: | ||
===Storage=== | ===Storage=== | ||
| − | Never store EGDN! | + | Never store EGDN! Use it as soon as possible. |
===Disposal=== | ===Disposal=== | ||
| + | EGDN can be neutralized by strongly dilute it in an organic solvent, then carefully burning the extremely diluted solution. | ||
| + | |||
| + | Diluted cold sodium hydroxide solution can also be used to neutralize diluted EGDN. | ||
==References== | ==References== | ||
| Line 26: | Line 139: | ||
Chemistry and Technology of Explosives - Volume II, first edition, 1965. | Chemistry and Technology of Explosives - Volume II, first edition, 1965. | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=5057 Playing with EGDN] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
[[Category:Organic compounds]] | [[Category:Organic compounds]] | ||
| − | |||
[[Category:Nitrates]] | [[Category:Nitrates]] | ||
[[Category:Nitrated organic compounds]] | [[Category:Nitrated organic compounds]] | ||
| + | [[Category:Energetic materials]] | ||
| + | [[Category:High explosives]] | ||
| + | [[Category:Liquids]] | ||
Latest revision as of 20:03, 25 October 2020
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
| |
| Names | |
|---|---|
| IUPAC name
1,2-dinitroxyethane
| |
| Other names
1,2-Bis(nitrooxy)ethane
1,2-Ethanediol dinitrate Dinitroglycol EGDN Ethylene dinitrate Ethylene glycol dinitrate Ethylene nitrate Ethane-1,2-diyl dinitrate Glycol dinitrate Nitroglycol | |
| Properties | |
| C2H4N2O6 | |
| Molar mass | 152.1 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | Odorless |
| Density | 1.4918 g/cm3 (20 °C) |
| Melting point | −22.3 °C (−8.1 °F; 250.8 K) |
| Boiling point | 197.5 °C (387.5 °F; 470.6 K) |
| 0.5 g/100 ml | |
| Solubility | Miscibile with glacial acetic acid, acetone, benzene, CCl4, chloroform, diethyl ether, methanol, toluene |
| Vapor pressure | 0.05 mmHg (20 °C) |
| Thermochemistry | |
| Hazards | |
| Safety data sheet | cpcb |
| Flash point | 215 °C (419 °F, 488 K) |
| Related compounds | |
| Related compounds
|
Methyl nitrate Nitroglycerin |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
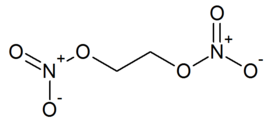
Ethylene glycol dinitrate, IUPAC name 1,2-dinitroxyethane, also known as nitroglycol or EGDN, is a pale yellow, syrupy, explosive liquid. In the series of alkyl nitrates it is among those which have a 1:1 ratio of nitroxy groups per carbon atom, and also exhibits a perfect oxygen balance.
Contents
Properties
Explosive
Thanks to its perfect oxygen balance, EGDN is one of the strongest explosives known, with a lead block test value of 650 cm3 (10% higher than NG). It is more sensitive to initiation but can also detonate incompletely with low velocity. The reported detonation velocity is around 7800 m/s.
Physical
Its properties are very similar to nitroglycerin. It is however less viscous, less dense and more volatile. The freezing point is -22 °C, and it has therefore been used for dynamites in cold climates.
Preparation
EGDN can be prepared by nitrating ethylene glycol under careful monitoring.
Another route is the reaction between ethylene oxide and dinitrogen pentoxide, in the presence of ozone. Reaction takes place in dichloromethane for 5 min, at temperatures between 10-15 °C. Yield is 96%.[1][2]
Handling
Safety
Like nitroglycerin, EGDN is highly explosive, although somewhat less sensitive to impact. One disadvantage EGDN has against NG is that EGDN is more volatile. This makes a person working with it more susceptible to its vasodilation effects, which is where the nitro group in the nitrated alcohol causes the body to widen blood vessels, resulting in phenomenal headaches.
Storage
Never store EGDN! Use it as soon as possible.
Disposal
EGDN can be neutralized by strongly dilute it in an organic solvent, then carefully burning the extremely diluted solution.
Diluted cold sodium hydroxide solution can also be used to neutralize diluted EGDN.
References
- ↑ Golding, P.; Millar, R. W.; Paul, N. C.; Richards, D. H.; Tetrahedron Letters; vol. 29; nb. 22; (1988); p. 2731 - 2734
- ↑ Golding, Peter; Millar, Ross W.; Paul, Norman C.; Richards, David H.; Tetrahedron; vol. 49; nb. 32; (1993); p. 7037-7050
Chemistry and Technology of Explosives - Volume II, first edition, 1965.