Difference between revisions of "Alpha-pinene"
(Fixed grammar and added SMILES code.) |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 5: | Line 5: | ||
| PIN = | | PIN = | ||
| SystematicName = | | SystematicName = | ||
| − | | OtherNames = α-Pinene | + | | OtherNames = α-Pinene<br>Pinene |
<!-- Images --> | <!-- Images --> | ||
| ImageFile = Alpha-pinene.png | | ImageFile = Alpha-pinene.png | ||
| − | | ImageSize = | + | | ImageSize = 250 |
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| Line 43: | Line 43: | ||
| 3DMet = | | 3DMet = | ||
| Abbreviations = | | Abbreviations = | ||
| − | | SMILES = | + | | SMILES = CC1=CCC2CC1C2(C)C |
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| Line 61: | Line 61: | ||
| MeltingPt_ref = | | MeltingPt_ref = | ||
| MeltingPt_notes = | | MeltingPt_notes = | ||
| + | | Odor = Pine, turpentine-like | ||
| pKa = | | pKa = | ||
| pKb = | | pKb = | ||
| − | | Solubility = | + | | Solubility = 0.000249 g/100 ml (25 °C) |
| − | | SolubleOther = Miscible with glacial [[acetic acid]], [[acetone]], [[ethanol]], [[isopropanol]] | + | | SolubleOther = Miscible with glacial [[acetic acid]], [[acetone]], [[chloroform]], [[diethyl ether]], [[ethanol]], [[isopropanol]]<br>Almost insoluble in [[glycerol]], [[propylene glycol]] |
| Solvent = | | Solvent = | ||
| − | | VaporPressure = | + | | VaporPressure = 4.75 mmHg at 25 °C |
}} | }} | ||
| Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| Line 87: | Line 88: | ||
}} | }} | ||
| Section6 = {{Chembox Hazards | | Section6 = {{Chembox Hazards | ||
| − | | AutoignitionPt = | + | | AutoignitionPt = 255 °C (491 °F; 528 K) |
| ExploLimits = | | ExploLimits = | ||
| − | | ExternalMSDS = | + | | ExternalMSDS = [https://www.docdroid.net/E5qDxMU/alpha-pinene-sa.pdf.html Sigma-Aldrich] |
| FlashPt = 33 °C (91 °F; 306 K) | | FlashPt = 33 °C (91 °F; 306 K) | ||
| LD50 = | | LD50 = | ||
| Line 104: | Line 105: | ||
| OtherFunction = | | OtherFunction = | ||
| OtherFunction_label = | | OtherFunction_label = | ||
| − | | OtherCompounds = | + | | OtherCompounds = [[Limonene]] |
}} | }} | ||
}} | }} | ||
| Line 111: | Line 112: | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| + | α-pinene and related compounds are commonly utilized in the fragrance industry, and as such, it is a precursor to many of these compounds. Hydration to [[α-terpineol]] can be accomplished by the reflux of α-pinene with aqueous [[sulfuric acid]] and [[acetone]] for a few hours, or by the action of concentrated sulfuric acid in [[ethanol]]]. The ester α-terpinyl acetate can be produced by esterification with glacial [[acetic acid]]. It can also be rearranged into [[camphene]] by strong acid catalysis in glacial acetic acid as a step in the production of camphor. With dilute acids, terpin hydrate becomes the major product. | ||
| − | + | Addition of [[iodine]] or [[phosphorus trichloride]] causes aromatisation, leading to p-cymene. | |
| − | + | ||
| + | ===Physical=== | ||
α-pinene is a lightweight, clear, colorless liquid in pure form, nearly insoluble in water but miscible with organic solvents. The odor of commercial α-pinene depends on its source; as it can be separated from turpentine by distillation, the odor often matches this. α-pinene boils at 155 °C (311 °F) and is only mildly flammable. | α-pinene is a lightweight, clear, colorless liquid in pure form, nearly insoluble in water but miscible with organic solvents. The odor of commercial α-pinene depends on its source; as it can be separated from turpentine by distillation, the odor often matches this. α-pinene boils at 155 °C (311 °F) and is only mildly flammable. | ||
==Availability== | ==Availability== | ||
| − | α-pinene is readily available as one of the most major | + | α-pinene is readily available as one of the most major constituents of turpentine and can be distilled in reasonably pure form from it. Turpentine is available as a solvent and paint stripper at many hardware and department stores. |
==Preparation== | ==Preparation== | ||
| Line 131: | Line 133: | ||
===Storage=== | ===Storage=== | ||
| − | Due to its volatility, α-pinene should be kept in airtight containers away from sources of open | + | Due to its volatility, α-pinene should be kept in airtight containers away from sources of open flames. |
===Disposal=== | ===Disposal=== | ||
| − | α-pinene is commonly encountered in biological systems and does not pose a significant threat to the environment nor to humans. Given its low density and immiscibility with water, pouring it down a sink may not be a good course of action for disposal, with an outdoor trash can presenting a better option. However it's best to have it soaked in a porous material, like sand or charcoal, to reduce any leaks on the ground or pavement. | + | α-pinene is commonly encountered in biological systems and does not pose a significant threat to the environment nor to humans. Given its low density and immiscibility with water, pouring it down a sink may not be a good course of action for disposal, with an outdoor trash can presenting a better option. However, it's best to have it soaked in a porous material, like sand or charcoal, to reduce any leaks on the ground or pavement. |
==References== | ==References== | ||
Latest revision as of 23:53, 25 August 2020
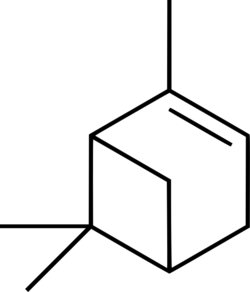
| |
| Names | |
|---|---|
| IUPAC name
(1S,5S)-2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene ((−)-α-Pinene)
| |
| Other names
α-Pinene
Pinene | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C10H16 | |
| Molar mass | 136.24 g/mol |
| Appearance | Colorless liquid |
| Odor | Pine, turpentine-like |
| Density | 0.858 g/cm3 (at 20 °C) |
| Melting point | −64 °C (−83 °F; 209 K) |
| Boiling point | 155 °C (311 °F; 428 K) |
| 0.000249 g/100 ml (25 °C) | |
| Solubility | Miscible with glacial acetic acid, acetone, chloroform, diethyl ether, ethanol, isopropanol Almost insoluble in glycerol, propylene glycol |
| Vapor pressure | 4.75 mmHg at 25 °C |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 33 °C (91 °F; 306 K) |
| Related compounds | |
| Related compounds
|
Limonene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
α-pinene(also written alpha-pinene) is an organic compound with chemical formula C10H16 belonging to the terpenes, a group of biologically important hydrocarbons. It is the most commonly encountered and well researched of the two pinene isomers, and has a number of niche uses in organic synthesis.
Contents
Properties
Chemical
α-pinene and related compounds are commonly utilized in the fragrance industry, and as such, it is a precursor to many of these compounds. Hydration to α-terpineol can be accomplished by the reflux of α-pinene with aqueous sulfuric acid and acetone for a few hours, or by the action of concentrated sulfuric acid in ethanol]. The ester α-terpinyl acetate can be produced by esterification with glacial acetic acid. It can also be rearranged into camphene by strong acid catalysis in glacial acetic acid as a step in the production of camphor. With dilute acids, terpin hydrate becomes the major product.
Addition of iodine or phosphorus trichloride causes aromatisation, leading to p-cymene.
Physical
α-pinene is a lightweight, clear, colorless liquid in pure form, nearly insoluble in water but miscible with organic solvents. The odor of commercial α-pinene depends on its source; as it can be separated from turpentine by distillation, the odor often matches this. α-pinene boils at 155 °C (311 °F) and is only mildly flammable.
Availability
α-pinene is readily available as one of the most major constituents of turpentine and can be distilled in reasonably pure form from it. Turpentine is available as a solvent and paint stripper at many hardware and department stores.
Preparation
α-pinene is more likely to be extracted from turpentine rather than synthesized in the home lab.
Projects
- Homemade perfumes and fragrances
Handling
Safety
While not strongly toxic, α-pinene produces vapors that can cause respiratory problems and may act as a skin irritant as well. It should not be used near open flame due to its flammability.
Storage
Due to its volatility, α-pinene should be kept in airtight containers away from sources of open flames.
Disposal
α-pinene is commonly encountered in biological systems and does not pose a significant threat to the environment nor to humans. Given its low density and immiscibility with water, pouring it down a sink may not be a good course of action for disposal, with an outdoor trash can presenting a better option. However, it's best to have it soaked in a porous material, like sand or charcoal, to reduce any leaks on the ground or pavement.
References
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Biologically-derived compounds
- Fragrant compounds
- Terpenes
- Hydrocarbons
- Liquids